Exploring Atoms, Elements, and the Periodic Table
Delve into the fascinating world of atoms and the periodic table with experiments on magnesium and zinc oxides. Discover how different elements combine to form compounds and analyze their composition. Learn about the relative atomic weights according to historical figures like John Dalton and Amadeo Avogadro, and explore Dmitri Mendeleev's groundbreaking periodic table of elements.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
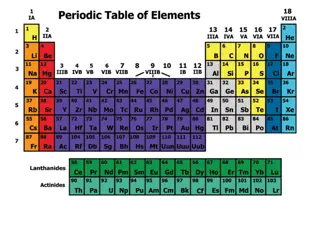
The Discovery of Atoms and The Development of the Periodic Table
Magnesium and Magnesium Oxide Name: _____________________________ Aim: To produce magnesium oxide from magnesium (metal) and to analyse its composition. Equipment: crucible and lid, tongs, pipe-clay triangle, Bunsen burner, heat-proof mat, magnesium ribbon (5-10 cm long), electronic balance. Method: 1.Record the mass of the crucible and its lid: __________________ 2.Record the mass of the strip of magnesium : ___________________ 3.Curl up the magnesium ribbon and heat it strongly in the crucible. Keep the lid on the crucible. The magnesium will soon start to burn. Ensure that it doesn t burn too quickly by keeping the lid on. 4.After all the magnesium metal has been completely transformed into magnesium oxide, reweigh the crucible, its lid, and the MgO Results: Mass of Mg Mass of Crucible and Lid Mass of crucible + lid + Mg Mass of crucible + lid + MgO Mass of MgO Overall increase in mass (mass of oxygen) Describe what happened when the magnesium was heated: _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ Analysis: _________ g Mg + __________g O2 __________ MgO % (by weight) of Mg atoms in MgO: % (by weight) of O atoms in MgO:
Zinc Oxide, ZnO % O = 19.7% 80.3% % Zn = 124.47g ZnO 100g Zn + 24.47g O2 % Zn % O x100%= 19.7% 100g Zn 124.47g ZnO 24.47g O 124.47g ZnO = 80.3% x100%
Tin Oxide(s) 113.5gstannous 100g tin + 13.5g oxygen oxide stannum = tin (Sn) SnO 127gstannic + 27g oxygen 100g tin oxide SnO2
Atom Relative Atomic Weight (according to Dalton) Relative Atomic Weight (actual) John Dalton Hydrogen Carbon 1 5 1 12 Nitrogen 5.4 14 J ns Berzelius Atom Oxygen Phosphorus 7 9 16 31 Sulphur 13 32 Copper 56 64 Amadeo Avogadro
Dmitri Mendeleevs Periodic Table of the Elements Gruppe I. Gruppe II. Gruppe III. Gruppe IV Gruppe V Gruppe VI Gruppe VII Gruppe VIII Reihen R2O RO RH4 RO2 RH3 R2O5 RH2 RO3 RH R2O7 RO4 R2O3 1 H=1 2 Li=7 Be=9,4 B=11 C=12 N=14 O=16 F=19 3 Na=23 Mg=24 Al=27,8 Si=28 P=31 S=32 CI=35,5 4 K=39 Ca=40 =44 Ti=48 V=51 Cr=52 Mn=55 Fe=56 Co=59 Ni=59, Cu=63. 5 (Cu=63) Zn=65 =68 =72 As=75 So=78 Br=80 6 Rb=85 Sr=87 ?Yt=88 Zr=90 Nb=94 Mo=96 =100 Ru=104, Rh=104, Pd=105, Ag=108, 7 (Ag=108) Cd=112 In=113 Sn=118 Sb=122 To=125 J=127 8 Ce=133 Ba=137 ?Di=138 ?Ce=140 9 ( ) 10 ?Er=178 ?La=180 Ta=182 W=184 Os=195, Ir=197, Pt=198, Au=199. 11 (Au=199) Hg=200 Ti=204 Pb=207 Bi=208 12 Th=231 U=240
Dmitri Mendeleevs Periodic Table of the Elements Gruppe I. Gruppe II. Gruppe III. Gruppe IV Gruppe V Gruppe VI Gruppe VII Gruppe VIII Reihen R2O RO RH4 RO2 RH3 R2O5 RH2 RO3 RH R2O7 RO4 R2O3 1 H=1 2 Li=7 Be=9,4 B=11 C=12 N=14 O=16 F=19 3 Na=23 Mg=24 Al=27,8 Si=28 P=31 S=32 CI=35,5 4 K=39 Ca=40 =44 Ti=48 V=51 Cr=52 Mn=55 Fe=56 Co=59 Ni=59, Cu=63. 5 (Cu=63) Zn=65 =68 =72 As=75 So=78 Br=80 6 Rb=85 Sr=87 ?Yt=88 Zr=90 Nb=94 Mo=96 =100 Ru=104, Rh=104, Pd=105, Ag=108, 7 (Ag=108) Cd=112 In=113 Sn=118 Sb=122 To=125 J=127 8 Ce=133 Ba=137 ?Di=138 ?Ce=140 9 ( ) 10 ?Er=178 ?La=180 Ta=182 W=184 Os=195, Ir=197, Pt=198, Au=199. 11 (Au=199) Hg=200 Ti=204 Pb=207 Bi=208 12 Th=231 U=240
Group 1 KEY Mendeleev s Periodic Table Period 2 Period 1 Element symbol: Relative atomic weight: H 1 H 1 He 4 H2O (2:1) Group 2 Group 3 Group 4 Group 5 Group 6 Group 7 Li 7 Be 9 B 11 C 12 N 14 O 16 - F Ne 20 19 - Li2O (2:1) BeO (1:1) B2O3 (2:3) CO2 (1:2) N2O5 (2:5) Period 3 Ar 40 Mg 24 Al Si 28 P 31 S Cl Na 23 27.8 32 35.5 Na2O (2:1) MgO (1:1) Al2O3 (2:3) SiO2 (1:2) SO3 (1:3) Cl2O7 (2:7) P2O5 (2:5) Period 5 selenium Se arsenic As 75 78 zinc Zn 65 As2O5 (2:5) SeO3 (1:3) ZnO (1:1) zinc oxide, ZnO arsenic pentoxide, As2O5 selenium trioxide, SeO3
Group 1 Key: KEY Mendeleev s Periodic Table Period 2 Period 1 Element symbol: Relative atomic weight: H 1 H 1 He 4 H2O (2:1) Group 2 Group 3 Group 4 Group 5 Group 6 Group 7 Li 7 Be 9 B 11 C 12 N 14 O 16 - F Ne 20 19 - Li2O (2:1) BeO (1:1) B2O3 (2:3) CO2 (1:2) N2O5 (2:5) Period 3 Ar 40 Mg 24 Al Si 28 P 31 S Cl Na 23 27.8 32 35.5 Na2O (2:1) MgO (1:1) Al2O3 (2:3) SiO2 (1:2) SO3 (1:3) Cl2O7 (2:7) P2O5 (2:5) Period 5 selenium Se 78 SeO3 (1:3) zinc Zn 65 arsenic As 75 As2O5 (2:5) eka- ekasilicon aluminium 68 72 ZnO (1:1) eka = one in Sanskrit
Dmitri Mendeleevs Periodic Table of the Elements Gruppe I. Gruppe II. Gruppe III. Gruppe IV Gruppe V Gruppe VI Gruppe VII Gruppe VIII Reihen R2O RO RH4 RO2 RH3 R2O5 RH2 RO3 RH R2O7 RO4 R2O3 1 H=1 2 Li=7 Be=9,4 B=11 C=12 N=14 O=16 F=19 3 Na=23 Mg=24 Al=27,8 Si=28 P=31 S=32 CI=35,5 4 K=39 Ca=40 =44 Ti=48 V=51 Cr=52 Mn=55 Fe=56 Co=59 Ni=59, Cu=63. 5 (Cu=63) Zn=65 =68 =72 As=75 So=78 Br=80 6 Rb=85 Sr=87 ?Yt=88 Zr=90 Nb=94 Mo=96 =100 Ru=104, Rh=104, Pd=105, Ag=108, 7 (Ag=108) Cd=112 In=113 Sn=118 Sb=122 To=125 J=127 8 Ce=133 Ba=137 ?Di=138 ?Ce=140 9 ( ) 10 ?Er=178 ?La=180 Ta=182 W=184 Os=195, Ir=197, Pt=198, Au=199. 11 (Au=199) Hg=200 Ti=204 Pb=207 Bi=208 12 Th=231 U=240
Group 1 Element name: Element symbol: Relative atomic weight: Hydrogen H 1 H2O (2:1) ? ? Hydrogen H 1 Helium He 4 Mendeleev s Periodic Table 1870s hydrogen fluoride, HF water, H2O methane, CH4 ammonia, NH3 Lithium Li 7 Li2O (2:1) ? ? Sodium Na 23 Na2O (2:1) ? ? Potassium K 39 K2O (2:1) ? ? Beryllium Be 9 BeO (1:1) ? Boron B 11 B2O3 (2:3) ? ?=1? Aluminium Al 27.8 Al2O3 (2:3) ? ?=1? Carbon C 12 CO2 (1:2) ? Nitrogen N 14 N2O5 (2:5) ? ?=?? Phosphorus P 31 P2O5 (2:5) ? ?=?? Oxygen O 16 - - Sulphur S 32 Fluorine F 19 - - Chlorine Cl 35.5 Neon Ne 20 ? ? Argon Ar 40 Magnesium Mg 24 MgO (1:1) ? Silicon Si 28 SiO2 (1:2) ? SO3 (1:3) 3 Cl2O7 (2:7) ? ?=?? ? ? ? Calcium Ca 40 CaO (1:1) ? hydrogen sulphide, H2S hydrogen chloride, HCl silane, SiH4 phosphine, PH3
Dmitri Mendeleevs Periodic Table of the Elements Gruppe I. Gruppe II. Gruppe III. Gruppe IV Gruppe V Gruppe VI Gruppe VII Gruppe VIII Reihen R2O RO RH4 RO2 RH3 R2O5 RH2 RO3 RH R2O7 RO4 R2O3 1 H=1 2 Li=7 Be=9,4 B=11 C=12 N=14 O=16 F=19 3 Na=23 Mg=24 Al=27,8 Si=28 P=31 S=32 CI=35,5 4 K=39 Ca=40 =44 Ti=48 V=51 Cr=52 Mn=55 Fe=56 Co=59 Ni=59, Cu=63. 5 (Cu=63) Zn=65 =68 =72 As=75 So=78 Br=80 6 Rb=85 Sr=87 ?Yt=88 Zr=90 Nb=94 Mo=96 =100 Ru=104, Rh=104, Pd=105, Ag=108, 7 (Ag=108) Cd=112 In=113 Sn=118 Sb=122 To=125 J=127 8 Ce=133 Ba=137 ?Di=138 ?Ce=140 9 ( ) 10 ?Er=178 ?La=180 Ta=182 W=184 Os=195, Ir=197, Pt=198, Au=199. 11 (Au=199) Hg=200 Ti=204 Pb=207 Bi=208 12 Th=231 U=240
Property Predicted Properties for Ekaaluminium, Ea Actual Properties of Gallium, Ga Atomic Weight 68 70 5.9 g/cm3 4.7 g/cm3 Density 6.0 grams/cm3 Dmitri Mendeleev Paul-Emile Lecoq Melting Point Low 30 C Oxide Formula Ea2O3 Ga2O3 Oxide s Density 5.5 grams/cm3 5.9 g/cm3 Gallium, Ga
Densities of the Elements 25 20 Density (g/cm3) 15 10 5 0 H Li B N F Na Al P Cl K Sc V Mn Co Cu Ga As Br Rb Y Nb Tc Rh Ag In Sb I Cs La Pr Pm Eu Tb Ho Tm Lu Ta Re Ir Au Tl Bi At Fr Ac Pa Element
Densities of the Elements (known in 1871) 25 20 Density (g/cm3) 15 densityarsenic = 5.7 g/cm3 densityzinc = 7.1 g/cm3 10 5 0 Te Ti Ni Tb Tl Th Na Er Ta Hg B Nb Ho H F P S K V Zr I Ir Li C N Zn Y Pd In W Pt Pb U O La Po Ac Mg Al Si As Ag Os Bi Cr Mn Br Rb Sr Mo Ru Rh Sn Sb Ba Au Ra Be Cl Fe Se Cs Ce Co Cu Cd Ca Element