Assay of Ascorbic Acid (Vitamin C) - Method and Procedure
Ascorbic acid, known as Vitamin C, is a crucial organic compound with antioxidant properties. This article discusses the assay of ascorbic acid using a redox titration method with potassium iodate and iodide. The principle, procedure, and calculations involved in determining the concentration of Vit
1 views • 8 slides
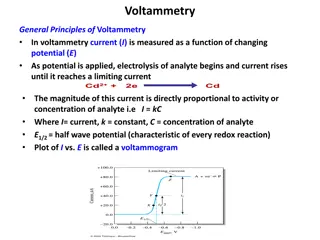
Understanding Voltammetry: Principles and Applications
In voltammetry, current is measured as a function of changing potential. The magnitude of current is directly proportional to the activity or concentration of the analyte. A voltammogram is plotted between current and potential, showing the characteristic half-wave potential. The process involves a
6 views • 76 slides
Advancements in Chemical Mechanisms for Air Quality Management
Daniel Jacob and team have been enhancing chemical mechanisms in the GEOS-Chem model to support US air quality management. Ongoing work includes developing new mechanisms for aromatic VOCs, tropospheric halogens, mercury redox, adaptive mechanism reduction, machine learning applications, and unifica
0 views • 19 slides
Chemical Analysis and Redox Reactions in Chemistry
Iron(II) ethanoate concentration determination using redox titration with cerium(IV) sulfate, balanced redox equations for manganate(VII) ion oxidizing iron(II) ion, and calculations of iron percentage in samples using titration with potassium manganate(VII). Molarities and concentrations are calcul
1 views • 6 slides
Understanding Redox Reactions in Chemistry
Salts can be prepared through redox reactions involving metals and acids. This interactive lesson covers oxidation numbers, identifying oxidized and reduced elements, and explaining electron transfer in redox reactions. Examples include reactions of aluminum with sulfuric acid and magnesium with cop
2 views • 12 slides
Dakin Rearrangement in Organic Chemistry: Mechanism and Positional Effects
The Dakin Rearrangement, also known as Dakin oxidation, is an organic redox reaction involving hydroxylated phenyl aldehydes or ketones reacting with hydrogen peroxide to form benzenediols and carboxylates. The mechanism includes nucleophilic addition, [1,2]-aryl migration, and final product formati
1 views • 9 slides
Understanding Enemark-Feltham Notation in Iron-Nitrosyl Complexes
Iron-Nitrosyl complexes are redox non-innocent, with NO exhibiting multiple redox states. Enemark-Feltham Notation helps in determining metal-ligand interactions and oxidation states. Detailed information on NO ligands, bonding characteristics, and methods for analyzing iron-NO systems are discussed
0 views • 6 slides
Chemical Reactions and Energy Transfers
Understanding chemical reactions involving thermal decomposition of metal carbonates, identifying exothermic and endothermic reactions based on energy transfers, and recognizing oxidation and reduction in redox reactions.
1 views • 5 slides
Understanding Soil Chemistry and Redox Reactions in Environmental Chemistry
Soil chemistry plays a crucial role in sustaining healthy soils by influencing nutrient availability through oxidation and reduction processes. Redox reactions in soil are impacted by factors like oxygen content and water presence, affecting nutrient supplies. The redox status of soil reflects its n
1 views • 92 slides
Redox Titration: Potassium Permanganate with Iron(II) Salt Assay
Ferrous sulfate (FeSO4.7H2O) is used in medical treatments for iron deficiency. This article discusses the redox titration process involving potassium permanganate and ferrous sulfate, along with the chemical principles, procedure, and calculations involved. Potassium permanganate is a powerful oxid
5 views • 7 slides
Understanding Potentiometry and Electrochemical Cells
Electrochemical cells play a vital role in redox reactions, with potentiometry being a quantitative analysis method based on measuring potentials. These cells consist of two half-cells where reduction and oxidation take place, forming anode and cathode. Reference electrodes like calomel and Ag/AgCl
0 views • 9 slides
Understanding Redox Reactivity and Balancing Equations in Acidic Solutions
Exploring the concept of reactivity in redox reactions using zinc, nickel, and copper, followed by a detailed guide on balancing redox equations in acidic solutions. Learn how to determine oxidation numbers, identify redox atoms, balance charges, and handle oxygen and hydrogen atoms to achieve balan
0 views • 18 slides
Understanding Cyclic Voltammetry in Electrochemical Analysis
Cyclic voltammetry is a crucial electroanalytical technique for studying electrochemical behavior. It involves sweeping potential in a cyclic manner to measure current responses, aiding in understanding redox processes, electron transfer kinetics, and coupled reactions. The technique requires carefu
0 views • 10 slides
Microbiological Inspection of Mineral Water by Redox Potential Measurement
MicroTester is a validated method for rapid microbiological testing of various types of water such as mineral water and carbonated water. Real-time monitoring of microbial properties in water production is crucial for ensuring safety and quality. The energy for microbial growth comes from biological
0 views • 27 slides
Global Flow Battery Market, Projected to Reach $1.03 Billion by 2031
Flow Battery Market by Offering (Energy Storage Systems), Battery Type (Vanadium Redox Flow Batteries, Zinc-Bromine Flow Batteries), Material, Ownership, Application, End User (Utilities, Commercial & Industrial), and Geography - Global Forecast to 2
1 views • 4 slides
Understanding Oxidation-Reduction Reactions in Chemistry
Explore the concept of oxidation and reduction in chemistry, which are fundamental processes that occur simultaneously in oxidation-reduction reactions. Learn about the role of oxygen, different types of oxidation reactions beyond burning, such as bleaching stains, and the concept of reduction invol
0 views • 34 slides
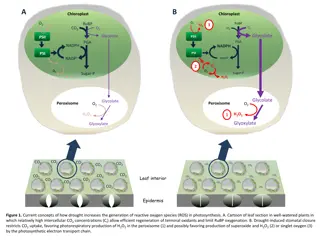
Understanding Drought-Induced Reactive Oxygen Species Generation in Photosynthesis
Leaf responses to drought conditions leading to increased generation of reactive oxygen species (ROS) in photosynthesis are explained. Drought-induced stomatal closure affects CO2 uptake, promoting H2O2 production in peroxisomes and potential ROS production by the photosynthetic electron transport c
0 views • 5 slides
Feasibility of Using Fe0 to Remediate Groundwater Lead Pollution in an Abandoned Tailings Dam
Heavy metal pollution in soil and groundwater from an abandoned tailings dam poses a persistent challenge. This study explores using iron (Fe0) in a Permeable Reactive Barrier (PRB) to remove lead (Pb) via redox reaction. The contaminated area, impacted by lead pollution, underwent experiments to as
0 views • 17 slides
Chemistry Revision Mind Map Unit 1 Summary
Exploring Unit 1 of Chemistry revision, we delve into bonding of the first 20 elements, trends in the periodic table, structure and bonding concepts, and oxidation and reduction reactions. Topics covered include melting points, boiling points, covalent radius, ionization energy, types of bonding, in
0 views • 4 slides
Understanding Electron Transport Chain and ATP Synthesis in Biochemistry
This course delves into the intricacies of electron transport and oxidative phosphorylation in biochemistry, elucidating how NADH and FADH2 are re-oxidized to generate ATP in eukaryotes and prokaryotes. It explores redox potential, oxidation-reduction reactions, and the role of standard redox potent
0 views • 20 slides
Study on Electrochemical Dissolution of Iron Monosulfide in Nuclear Waste Disposal
Exploration of the electrochemical dissolution of iron monosulfide in the context of nuclear waste disposal, focusing on its impact on redox potential, corrosion products, and transport of radionuclides. The project aims to clarify reaction kinetics, mechanisms of sulfur release, and factors control
0 views • 13 slides
Understanding Electrochemistry Concepts and Redox Reactions
Explore the fundamentals of electrochemistry, oxidation-reduction reactions, and identification of redox components. Learn about oxidation states, electron transfer, and half-reactions. Dive into examples and visual aids to grasp the concepts easily.
0 views • 42 slides
Understanding Electrochemistry: Redox Reactions, Cells, and Equations
Electrochemistry is a branch of chemistry that involves redox reactions, galvanic cells, standard reduction potentials, balancing redox equations, batteries, corrosion prevention, and electrolysis. Learn about the fundamental principles, examples of redox reactions, and how to balance equations usin
0 views • 61 slides
Understanding Metabolic Pathways and Regulatory Mechanisms
Metabolic pathways are a series of enzymatic reactions interconnected to form a flow of substrates and products. Irreversible steps, known as committed steps, determine pathway direction. Regulation, through positive or negative feedback, controls enzyme activity. Branched pathways share common inte
0 views • 20 slides
Understanding Cyclic Voltammetry in Electrochemical Methods
Electrochemical methods, such as cyclic voltammetry, are crucial for studying electron transfer processes, redox reactions, and adsorption on surfaces. Cyclic voltammetry involves varying the applied potential at a working electrode to monitor electron flow and chemical reactions. Peaks in the curre
0 views • 11 slides
Understanding Oxidation-Reduction Reactions in Analytical Chemistry
Oxidation-reduction reactions play a crucial role in various chemical processes, including photosynthesis and corrosion. This content delves into the basics of redox reactions, explaining how electrons are transferred between reactants, leading to changes in oxidation numbers. Examples such as the r
0 views • 10 slides
Geochemistry and Petrology of Anna Shale in Southwestern Illinois
This study by Jacob Dyson, Susan Rimmer, and Scott Erick explores the geochemistry and organic petrology of the Pennsylvanian Anna Shale in Southwestern Illinois. The research aims to assess source-rock quality, determine organic matter sources, evaluate paleo-redox conditions, and investigate trace
0 views • 15 slides
Bioenergetics Basics: Overview, Topics & Energy Diagrams
The content covers the fundamentals of bioenergetics, including topics like anabolism, catabolism, oxidation, reduction, redox coenzymes, substrate-level phosphorylation, oxidative phosphorylation, and photosynthesis. It also explores energy level diagrams, metabolism, oxidation-reduction reactions,
0 views • 21 slides
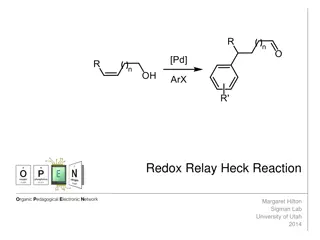
Understanding the Redox-Relay Heck Reaction in Organic Synthesis
The Redox-Relay Heck Reaction is a powerful tool in organic synthesis that allows for the functionalization of olefins with aryl groups. Developed by Sigman and colleagues, this reaction involves a palladium-catalyzed relay controlled by a thermodynamic sink, leading to the formation of aldehydes or
0 views • 6 slides
Redox Conditions and pH Control in a Mountain Watershed: Study in Red Canyon, Wyoming, USA
Exploring redox conditions and trace element concentrations in a semi-arid mountain watershed, this study in Red Canyon, Wyoming, delves into the impact of oxic surface water and anoxic groundwater on trace element cycling. The investigation aims to enhance understanding of seasonal variabilities, f
0 views • 11 slides
Understanding Electrochemical Systems and Processes
Electrochemical engineering involves the study of devices and processes that convert chemical energy to electrical energy through ionic conductors. This field explores redox reactions, energy-producing processes, electrocatalysis, anodic and cathodic reactions, and the interplay between thermochemic
0 views • 25 slides
Mössbauer Spectroscopy: Principles and Applications
Mössbauer Spectroscopy is a technique discovered by Rudolf Mössbauer in 1958, based on the recoilless emission and absorption of gamma radiation. It is used to identify minerals, determine redox states, quantify mineral abundancies, and assess crystallinity. The method involves analyzing typical s
0 views • 14 slides
Reactions of Alkenes: Strategies and Mechanisms
The excerpt provides detailed insights into reactions of alkenes, emphasizing main reaction classes, key characteristics, intermediate states, and specific mechanisms like bridgehead halohydrin. It covers important concepts such as carbocation addition, free radical addition, and organometallic/redo
0 views • 12 slides
Lemon-Powered Car Experiment: Creating Electricity from Citrus Cells
The Lemon-Powered Car experiment involves determining the potency of reducing agents, creating chemical cells to generate electricity, utilizing capacitors to store and release energy, and building a car powered by a chemical reaction. Key concepts covered include redox reactions, batteries, capacit
0 views • 21 slides
Assay of Ferrous Sulfate (FeSO4.7H2O) by Redox Titration Experiment
This experiment involves determining the weight and weight percentage of an unknown sample of FeSO4.7H2O through a redox titration using potassium permanganate solution. Ferrous sulfate, a chemical compound used in medical treatments, is oxidized to ferric sulfate in the presence of sulfuric acid. T
0 views • 9 slides
Understanding Electrochemistry: A Comprehensive Overview
Dive into the world of electrochemistry with this detailed review covering topics such as oxidation, reduction, half-reactions, and the significance of oxidation numbers in balancing redox equations. Explore key concepts like OIL RIG (Oxidation Is Loss, Reduction Is Gain) and learn how to apply elec
0 views • 17 slides
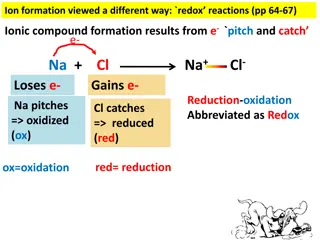
Understanding Redox Reactions and Ionic Compound Formation
Explore the concept of redox reactions through the process of ion formation, where elements lose or gain electrons to create ionic compounds. Learn about oxidation (ox) and reduction (red) in chemical reactions, and how to identify which elements lose or gain electrons based on charge changes. Disco
0 views • 14 slides
Understanding Redox Half Reactions: Assigning Oxidation Numbers and Half-Reactions
In this content, we explore assigning oxidation numbers to elements in compounds such as HClO, HClO2, HClO3, and HClO4. We then delve into the Haber Process to understand redox reactions. The concept of oxidation and reduction, as well as the significance of electrons in these reactions, is illustra
0 views • 20 slides