Understanding Redox Reactivity and Balancing Equations in Acidic Solutions
Exploring the concept of reactivity in redox reactions using zinc, nickel, and copper, followed by a detailed guide on balancing redox equations in acidic solutions. Learn how to determine oxidation numbers, identify redox atoms, balance charges, and handle oxygen and hydrogen atoms to achieve balanced equations like a pro.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Reactivity Tables (usually reducing) show relative reactivities: In the examples from the previous slide, the acid solution (H+) will react with anything below it in the Table but not above. Nickel and Zinc, .but not Copper.
Number of electrons gained must equal the number of electrons lost. - 2 e- +2 e- Use oxidation numbers to determine what is oxidized and what is reduced. +2 e- 0 0 Cu 2+ Cu (s) - 2 e- 2 H + H2(g) Refer to Balancing Oxidation-Reduction Reactions
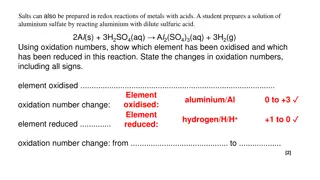
Balancing Redox Equations in acidic solutions 1) Determine the oxidation numbers of atoms in both reactants and products. 2) Identify and select out those which change oxidation number ( redox atoms) into separate half reactions . 3) Balance the redox atoms and charges (electron gain and loss must equal!). 4) In acidic reactions balance oxygen with water then hydrogen from water with acid proton(s). 1
Balancing Redox Equations in acidic solutions 1 Fe+2(aq)+ Cr2O72-(aq) +H+(aq) Fe3+(aq) + Cr3+(aq) + H2O(l) Fe 2+(aq)+ Cr2O72-(aq) +H+(aq) Fe 3+(aq) + Cr 3+(aq) + H2O(l) ? Cr oxidation number? x = ? Cr ; 2x+7(-2) = -2; x = +6
Balancing Redox Equations in acidic solutions 1 -e - Fe 2+(aq) Fe 3+(aq) Cr2O72-(aq) + Cr 3+(aq) Cr = (6+) 6 (Fe 2+(aq) -e - Fe3+(aq)) 6 Fe 2+(aq) 6 Fe3+(aq) + 6 e - Cr2O72-(aq) + 6 e - 2 Cr3+(aq) 2 6 e -
Balancing Redox Equations in acidic solutions 1 6 Fe2+(aq) 6 Fe3+(aq) + 6 e - Cr2O72-(aq) + 6 e - 2 Cr3+(aq) 6 Fe2+(aq)+ Cr2O72-(aq) + ? 2nd H+(aq) 6 Fe3+(aq) + 2 Cr3+(aq)+ ? 1st Oxygen H2O(l) Oxygen = 7 2nd (Hydrogen) = 14
Balancing Redox Equations in acidic solutions 1 Completely Balanced Equation: 6 Fe2+(aq)+ Cr2O72-(aq) + 14 H+(aq) 6 Fe3+(aq) + 2 Cr3+(aq)+ 7H2O(l)
Balancing Redox Equations in basic solutions 1 1) Determine oxidation numbers of atoms in Reactants and Products 2) Identify and select out those which change oxidation number into separate half reactions 3) Balance redox atoms and charges (electron gain and loss must equal!) 4) In basic reactions balance the Oxygen with hydroxide then Hydrogen from hydroxide with water
Balancing Redox Equations in basic solutions MnO2 (aq)+ ClO31-(aq) + OH 1-aq) MnO41- (aq)+ Cl 1-(aq) + H2O(l) Mn4+ (MnO2) Mn7+ (MnO4 ) 1- Cl+5 (ClO3 ) 1-+ 6 e- Cl 1-
Balancing Redox Equations in basic solutions Electronically Balanced Equation: 2 MnO2 (aq)+ ClO31-(aq) + 6e - 2 MnO4 1- + Cl 1- + 6 e-
Balancing Redox Equations in basic solutions Completely Balanced Equation: 2 MnO2 (aq)+ ClO31-(aq) + 2OH 1- (aq) 2 MnO4 (aq)1- + Cl 1- (aq)+ 1 H2O (l) 9 O in product