Understanding Redox Half Reactions: Assigning Oxidation Numbers and Half-Reactions
In this content, we explore assigning oxidation numbers to elements in compounds such as HClO, HClO2, HClO3, and HClO4. We then delve into the Haber Process to understand redox reactions. The concept of oxidation and reduction, as well as the significance of electrons in these reactions, is illustrated. Half-reactions are discussed in detail, emphasizing conservation of mass and charge. Through examples, the process of balancing half-reactions is explained, providing a comprehensive understanding of redox chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
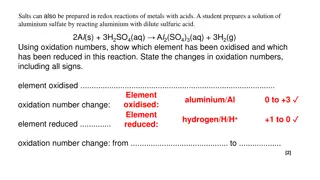
Topic: Redox Half reactions Assign Oxidation number to H, Cl and O for the following compounds 1. HClO 2. HClO2 3. HClO3 4. HClO4
Now we know how to assign oxidation number we can look at redox rxns Haber Process N2(g)+ 3H2(g) 2NH3(g) Start by assigning oxidation numbers 0 0 -3 +1 N2(g)+ 3H2(g) 2NH3(g) What was oxidized? Reduced?
0 0 +1 -3 N2(g) + 3H2(g) 2NH3(g) Oxidation Is Losing electrons O I L 0 -1 -2 -3 -4 4 3 2 1 Began Ended N 0 -3 Reduction Is Gaining electrons R I G Gained 3 electrons = Reduced H 0 +1 Lost 1 electron = Oxidized
Why use the word reduced when electrons are gained? Electrons are Negative! Look how oxidation number changes Ex: Cl gains an electron Cl-1 oxidation # from 0 to -1; the # was reduced
Half Reactions Even though oxidation & reduction reactions occur together we write separate equations for each process and include # of e- gained/lost known as Half-Reactions
Half Reactions Half-reactions must demonstrate: conservation of mass & conservation of charge # atoms on left must = # atoms on right (always balance mass first!!!!) Keep 7 diatomics together, everything else write as single ion) total charge on left must = total charge on right Add electrons to more positive side
0 0 -3 +1 N2 + 3H2 2NH3 Oxidation = electrons lost (becomes more positive) So electrons are on the product side H2 H+1 + 1e- 0 +1 1 and -1 equals 0 = OIL But something is wrong!
Remember H2 H+1 + 1e- Total Charge on left = Total Charge on right # atoms on left = # atoms on right H2 2H+1 + 1e- Something is still wrong! Charge is off now H2 2H+1 + 2e- 2 x +1 = +2 and -2 equals 0
0 0 -3 +1 N2 + 3H2 2NH3 Reduction = electrons gained (becomes more negative) So electrons are on the reactant side Each N gained 3 electrons, N2 + 3e- N-3 Balance mass But not you have -6 RIG 2 6
0 +2 -2 0 You Try: Mg + S MgS What is oxidized? What is reduced? Assign Oxidation Numbers Figure out change in oxidation numbers Mg: 0 to +2 = Oxidation S: 0 to -2 = Reduction
Now write the Half-Reactions 0 0 +2 -2 Mg + S MgS S is reduced: Mg is oxidized: Mg Mg+2 + 2e- S + 2e- S-2
0 0 +1 -1 +2 -1 Zn + 2HCl What is oxidized? Reduced? H2 + ZnCl2 Zn goes from 0 to +2 = oxidation H goes from +1 to 0 = reduction Cl goes from -1 to -1; No change
0 0 +1 -1 +2 -1 Zn + 2HCl H2 + ZnCl2 Write Half Reactions Zn 2H+1 + 2e- Zn+2 + 2e- H2
In Redox # of electrons gained has to equal number of electrons lost Zn Zn+2 + 2e- 2H+1 + 2e- H2 H2 2H+1 + 2e- N2 + 6e- 2N-3
In Redox # of electrons gained has to equal number of electrons lost H2 2H+1 + 2e- 3 3H2 6H+1 + 6e- N2 + 6e- 2N-3 3H2 +N2 6H+1 + 2N-3
N2(g) + 3H2(g) 2NH3(g) 3H2 +N2 6H+1 + 2N-3
The steps start with balanced rxn Assign oxidation numbers to all atoms in equation 1. 2. Determine elements changed oxidation number 3. Identify element oxidized & element reduced 4. Write half-reactions (diatomics must stay as is, everyone you can write with their oxidation number only NH3 = N-3 but Cl2 stays Cl2 not Cl) Make sure # of atoms on each side is balanced Make sure charge on each side is balanced 5. Number electrons lost & gained must be equal; multiply half-reactions if necessary 6. Add half-reactions
+2 +5 -2 +1 +5-2 0 0 Cu + AgNO3 2 Cu(NO3)2 + Ag 2 1. Assign oxidation numbers to all atoms in equation 2. Determine elements changed oxidation number Cu 0 +2 Ag +1 0 N +5 +5 unchanged O -2 -2 unchanged 3. Identify element oxidized & element reduced Oxidation (OIL) =Cu Reduction (RIG) = Ag
+2 +5 -2 +1 +5-2 0 0 Cu + 2AgNO3 Cu(NO3)2 + 2Ag 4. Write half-reactions Cu Cu+2 + 2e- Ag+1 + 1e- 5. Number electrons lost & gained must be equal; multiply half- reactions if necessary 2(Ag+1 + 1e- Ag) 2Ag+1 + 2e- 2Ag Ag
+2 +5 -2 +1 +5-2 0 0 Cu + 2AgNO3 Cu(NO3)2 + 2Ag 6. Add half-reactions; Transfer coefficients to skeleton equation Cu Cu+2 + 2e- 2Ag+1 + 2e- Cu + 2Ag+1 + 2e- 2Ag + Cu+2 + 2e- Cu + 2Ag+1 2Ag + Cu+2 +______________________ 2Ag Cu + AgNO3 Cu(NO3)2 + Ag 2 2