Bioenergetics Basics: Overview, Topics & Energy Diagrams
The content covers the fundamentals of bioenergetics, including topics like anabolism, catabolism, oxidation, reduction, redox coenzymes, substrate-level phosphorylation, oxidative phosphorylation, and photosynthesis. It also explores energy level diagrams, metabolism, oxidation-reduction reactions, and the calculation of oxidation numbers for atoms and molecules. Additionally, it provides resources for further study and visualization aids for understanding key concepts in bioenergetics.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
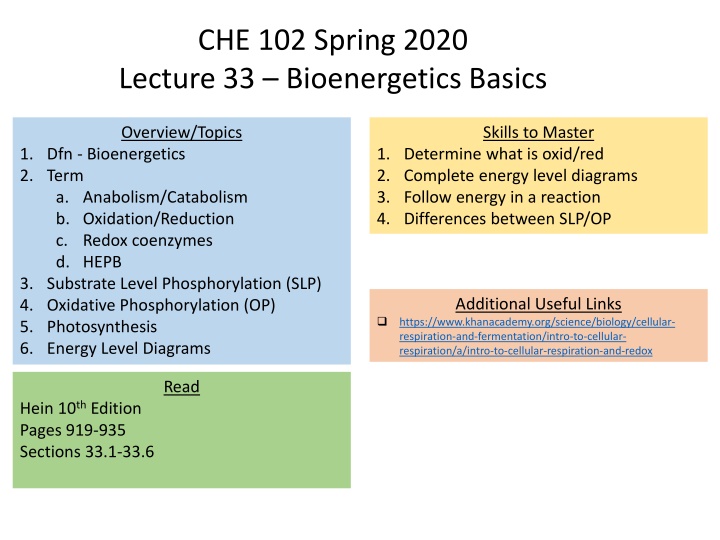
CHE 102 Spring 2020 Lecture 33 Bioenergetics Basics Overview/Topics Skills to Master 1. Dfn - Bioenergetics 2. Term a. Anabolism/Catabolism b. Oxidation/Reduction c. Redox coenzymes d. HEPB 3. Substrate Level Phosphorylation (SLP) 4. Oxidative Phosphorylation (OP) 5. Photosynthesis 6. Energy Level Diagrams 1. Determine what is oxid/red 2. Complete energy level diagrams 3. Follow energy in a reaction 4. Differences between SLP/OP Additional Useful Links https://www.khanacademy.org/science/biology/cellular- respiration-and-fermentation/intro-to-cellular- respiration/a/intro-to-cellular-respiration-and-redox Read Hein 10th Edition Pages 919-935 Sections 33.1-33.6
Can you: Identify and/or Draw a Saturated or Unsaturated FA? Structure -> Physical Properties -> Biological Property Cheat Sheet: You will have Table 28.1 on all exams
study of the transformation, distribution and utilization of energy by living organisms Bioenergetics 1. Photosynthesis 2. Embden-Meyerhof Pathway (EMP)/Glycolysis 3. Citric Acid Cycle (CAC) 4. -oxidation and Lipogenesis Can you: Definition Table 33.1 Big Picture
Metabolism sum of all the chemical reactions within a living organism Catabolism Anabolism 1. Complex Simple 2. Oxidize Carbon 3. Produces Energy 4. Examples a. EMP/Glycolysis (Ch. 34) b. CAC (Ch. 34) c. -oxidation (Ch. 35) 1. Simple Complex 2. Reduce Carbon 3. Consumes/Requires Energy 4. Examples a. Photosynthesis (Ch. 33) b. Gluconeogenesis (Ch. 34) c. Lipogenesis (Ch. 35) Can you: Definitions Recognize Anabolism and Catabolism Energy Level Diagrams
Review Oxidation and Reduction (Redox) *They are always paired Oxidation Reduction 1. LEO 2. Lose e- 3. Gain bonds to Oxygen 4. Lose bonds to Hydrogen 5. Release Energy 1. GER 2. Gain e- 3. Lose bonds to Oxygen 4. Gains bonds to Hydrogen 5. Store Energy Can you: Definitions Recognize Oxid. and Red. Energy Level Diagramsv
Can you: Calculate Ox # of atom Calculate average Ox # molecule Recognize Low/High Energy Oxidation Number (Ox #:) 1. Charge an atom would have it all the electrons in covalent bonds were instead treated like ionic bonds (gain/lost not shared) 2. More Reduced (more negative Ox #) High Energy 3. More Oxidized (more + Ox #) Low Energy 4. 4 Basic Rules a. Hydrogen always +1 b. Oxygen always -2 c. Like atoms bonds treated as 0 d. Molecules are neutral (ie sum Ox # s = 0 Carbohydrate Fatty Acid H H C C C O C O C C C OH H Ox #: +4 Ox #: -4 Ox #: -2 Ox #: +0 Low Energy High Energy
Can you: Calculate Ox # of atom Calculate average Ox # molecule Recognize Low/High Energy A couple more examples Which has more energy? Explain. a) 1-butanol b) 2-butanone c) Butanoic acid
Redox Coenzymes Enzymes used to move electrons/energy around (and store) Can you: Importance of Redox Coenzymes Recognize them in reactions Recognize Low/High Energy 1. 3 Main Redox Coenzymers a. NADH/NAD+ b. NADPH/NADP+ c. FADH2/FAD 2. Synthesized from vitamins (niacin and riboflavin) 3. Carry electrons to mitochondrial electron transport system 4. 85% of a cells energy from redox process s 5. Generally converted to and stored in HEPB (ATP)
NAD - Nicotinamide Adenosine Dinucleotide FAD Flavin Adenine Dinucleotide Low Energy High Energy Low Energy High Energy NADP: has PO4-2 group instead of OH Can you: Recognize Low/High energy
Close up on what changes NAD/NADPH FAD Reduced High Energy Oxidized Low Energy
Just Abbreviations Reduction NAD+ NAD Oxidation Reduction FAD FADH2 Oxidation
Can you: Recognize Low/High energy Determine Oxid/Red Complete AND Explain the problems You Try It! Add FAD and FADH2 to the reaction below. COO- COO- CH CH2 CH CH2 COO- COO- What is wrong with the following equation? O O O- O- C C + NAD+ + NADH H C OH C O CH3 CH3
You Try It! - Answers Add FAD and FADH2 to the reaction below. COO- COO- CH CH2 + FAD Oxidized coenzyme + FADH2 Reduced coenzyme CH CH2 COO- Oxidized organic molecule COO- Reduced organic molecule Explanation The molecule is being oxidized (it lost two bonds to H) therefore the redox coenzyme must be reduced (go from low energy to high energy)
You Try It! - Answers What is wrong with the following equation? O O O- O- C C + NAD+ oxidized coenzyme + NADH reduced coenzyme H C OH C O CH3 CH3 reduced organic molecule oxidized organic molecule Explanation In a redox reaction one molecule is oxidezed and one molecule must be reduced (they are paired). In this example, both the molecule (lost bond to oxygen/gained bond to H) and the redox coenzyme are being reduced (went from low energy to high energy, or gained a bond to H).
Review HEPB/ATP Dehydration -H2O Formation Reaction +H2O Hydrolysis 1. Easily accessible Dehydration/Hydrolysis 2. Universal all plants/animals use it Can you: Importance of HEPB and ATP ID Type of Reactions
Can you: Compare and Contrast SLP vs OP Energy Conversion 1. Energy from nutrients with reduced carbons (energy-yielding nutrients) is converted to high-energy phosphate bonds that are used to do work in the body 2. Two methods for storing energy in HEPB/phosphate anhydride bonds a. Substrate Level Phosphorylation (SLP) b. Oxidative Phosphorylation (OP)
Substrate Level Phosphorylation (SLP) 1. Anaerobic (No O2) 2. Occurs in Cytoplasm 3. Inefficient 4. Examples Glycolysis 5. Often transferred to ATP in a latter step Dehydration -H2O Can you: Complete the reaction ID HEPB
Oxidative Phosphorylation (OP) Can you: Complete the reaction Understand Energy Level Diagrams 1. Aerobic (O2) 2. Occurs in Mitochondria 3. Efficient 4. Examples CAC/ -oxidation 5. Redox Coenzymes converted to ATP
Can you: Calculate Oxid # s Anabolic or Catabolic? Photosynthesis 1. 1961 Noble Prize Melvin Calvin 2. Converting Sunlight to Reduced Carbons Light Reactions 6 CO2 + 6 H2O + 2820 kJ C6H12O6 + 6 O2 Dark Reactions
Photosynthesis Figure 33.9 CHE 112