Understanding Chemical Quantities: The Mole and Molar Mass
Explore the concept of chemical quantities through the mole and molar mass. Learn how to measure substances, calculate moles, find molar masses of compounds, and solve related problems in this informative chapter. Discover the significance of Avogadro's number, representative particles, and more in
7 views • 41 slides
Understanding Stoichiometry in Chemistry
Stoichiometry in chemistry involves calculating quantities of reactants and products in a chemical reaction based on a balanced equation. This process ensures the conservation of mass and atoms. Mole ratios are used as conversion factors, and steps such as converting to moles and applying the mole r
1 views • 25 slides
Understanding Mole Calculations in Chemistry
Explore various mole calculations in chemistry such as determining mass from moles, moles from mass, and comparing particles in different substances. Learn how to calculate the mass of substances, the number of particles, and perform calculations using balanced equations. Dive into concepts like mol
0 views • 49 slides
Understanding Gas Laws: Boyle's, Charles', Gay-Lussac's, and Avogadro's Laws
Gas laws such as Boyle's Law, Charles' Law, Gay-Lussac's Law, and Avogadro's Law govern the behavior of gases under different conditions. Boyle's Law relates pressure and volume at constant temperature, Charles' Law relates volume and temperature at constant pressure, Gay-Lussac's Law relates pressu
1 views • 19 slides
Understanding Moles and Formula Mass in Chemistry
Dive into the world of moles, Avogadro's number, and formula mass calculations in chemistry. Learn how to convert moles to grams, grams to moles, and determine the number of atoms using Avogadro's number. Explore the concepts of formula mass and percentage composition to understand the composition o
0 views • 19 slides
Automated Melanoma Detection Using Convolutional Neural Network
Melanoma, a type of skin cancer, can be life-threatening if not diagnosed early. This study presented at the IEEE EMBC conference focuses on using a convolutional neural network for automated detection of melanoma lesions in clinical images. The importance of early detection is highlighted, as exper
0 views • 34 slides
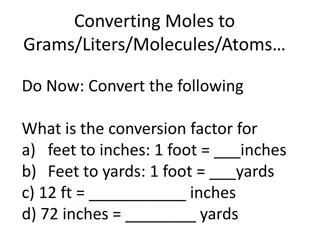
Converting Moles to Grams, Liters, Molecules, and Atoms
Explore the conversion factors for feet to inches and yards, and learn how to convert moles to grams, liters, molecules, and atoms using formula masses. Practice conversions and understand the relationship between moles, grams, and liters with helpful hints and examples.
0 views • 18 slides
Understanding Concentration in Solutions
Solutions involve the dissolution of solutes like salt or sugar in solvents such as water, resulting in different concentrations. This concentration can be expressed as moles per liter, known as molarity. By calculating the amount of substance in moles using solution volume and concentration, you ca
0 views • 8 slides
Experimental Method for Measuring Oxygen Production in Green Plants
The reporter proposed an experimental method to measure oxygen production by an aquatic plant, highlighting the process and outcomes. While there were detailed explanations on photosynthesis, the report lacked essential elements such as explaining the choice of plant and the source of carbon dioxide
1 views • 5 slides
Understanding Stoichiometry in Chemical Reactions
Stoichiometry is the concept of predicting the amounts of reactants and products in a chemical reaction, similar to following a recipe in cooking. It involves balancing chemical equations and determining the quantities of substances involved. By paying attention to coefficients, one can calculate ho
2 views • 65 slides
Integumentary System Assessment Techniques Lecture
Explore the physical assessment techniques for the integumentary system, focusing on the skin, hair, and nails. Learn about inspection and palpation methods, nail assessment criteria including shape variations, and skin assessment parameters such as color, texture, and pigmentation. Discover how to
0 views • 22 slides
Calculating Limiting Reagent in Chemical Reactions
Calculating the amount of reactants in excess and the limiting reagent plays a crucial role in determining the maximum extent of a chemical reaction. By using the relative numbers of moles of substances as shown in balanced equations, one can identify the reactant that is fully utilized, hence limit
0 views • 14 slides
Whispers of Deep Time: Reflections on Evolution and Nature
Wind whispers through the fossil record, carrying echoes of ancient lifeforms and primordial oceans. Soft, unbreakable creatures traverse the depths while desert floors tell stories of rolling processes and rain's wanderings. Between horizons of desert and grassland lie tales of early life and shore
0 views • 12 slides
Understanding Concentration of Solutions in Physiology
Concentration of solutions is crucial in understanding the properties of substances in Physiology. This involves concepts like percentage solutions and molar solutions, where the amount of solute is measured in grams or moles relative to the volume of the solution. Percentage solutions are commonly
0 views • 8 slides
Understanding Stoichiometry in Chemical Reactions
Stoichiometry in chemical reactions involves mass changes, limiting reactants, and calculating yields like moles and grams. Learn to solve problems involving different reactants and products, determining the quantities involved in a reaction. Examples cover decompositions, formations, and calculatio
0 views • 22 slides
Understanding Solution Concentration in Chemistry
Solutions in chemistry are homogeneous mixtures of two or more substances where each maintains its own chemical identity. They consist of a solute, the substance being dissolved, and a solvent, the substance used to dissolve the solute. Solution concentration can be described qualitatively as concen
0 views • 17 slides
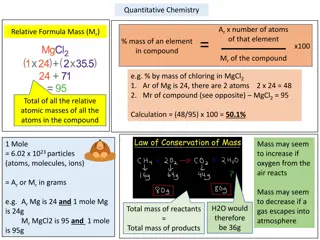
Understanding Quantitative Chemistry in Chemical Reactions
Explore the fundamentals of quantitative chemistry, from calculating percentages by mass to determining limiting reactants. Learn how to work out balanced symbol equations and solve concentration questions with ease. Enhance your knowledge of moles, molecular masses, and more in chemical reactions.
0 views • 4 slides
Understanding the Ideal Gas Law in Chemistry
Explore the concepts of the ideal gas law, including Avogadro's principle and gas stoichiometry at non-STP conditions. Learn how to apply the law to calculate pressure, volume, and moles of gases under various conditions with practical examples provided.
0 views • 13 slides
Investigating Ideal Gas Law: Pressure, Temperature, and Volume Relationship
Explore the relationship between pressure, temperature, volume, and number of gas molecules in a closed system through an experiment based on the Ideal Gas Law at the University of Ottawa's first-year physics laboratories. The experiment aims to determine if air behaves as an ideal gas and find the
0 views • 13 slides
Understanding Freezing Point Depression and Molality for Solutions
Introduction to molality and freezing point depression in solutions. Molality is a way to measure solution concentration, calculated using moles of solute and kilograms of solvent. By calculating the moles of NaCl in a salt solution and the mass of the solvent (ice/water), the molality can be determ
0 views • 9 slides
Understanding Percent Composition and Empirical Formulas
Explore the concept of percent composition, empirical formulas, and how to determine them through examples. Learn how to convert percentages to grams, calculate moles, and find the simplest ratio among elements in a compound.
0 views • 9 slides
Understanding Toxicity Through Stoichiometry and Molar Mass
Delve into the world of toxicity analysis by comparing the amounts of different substances using moles and molar mass. Explore the safety of sweeteners and learn how to utilize these concepts to assess toxicity levels. Engage in thought-provoking discussions and activities to deepen your understandi
0 views • 10 slides
Stoichiometry Calculations in Chemistry
Explore various stoichiometry calculations including determining the number of moles in given volumes and masses, calculating expected product masses based on balanced equations, and finding the mass of products produced in chemical reactions. Practice examples with magnesium, carbon dioxide, methan
0 views • 13 slides
Understanding Molar Mass and Avogadro's Number in Chemistry
Explore the concept of molar mass and Avogadro's number in chemistry through lessons on translating numbers into scientific notation, understanding moles, and finding molar mass on the periodic table. Discuss the relationship between mass and moles, differentiate between different quantities of a su
1 views • 11 slides
Understanding Mole Calculations and Its Applications
Dive into the world of mole calculations with Chapter 5 homework assignments, exploring concepts such as mole weight, the dozen concept, and sweet mole calculations. Discover the significance of moles in chemistry and how they relate to everyday scenarios like calculating the weight of eggs and unde
1 views • 28 slides
Understanding Moles and Molar Quantities in Chemistry
Chemistry involves understanding atomic structure, chemical equations, and measurement units like moles. Avogadro's number, elemental composition, and compound formulas play crucial roles in calculating and using substances in chemical reactions. Learn about the significance of mass numbers, moles,
0 views • 25 slides
Comprehensive Guide to Integumentary System Assessment Techniques
Learn the essential techniques for assessing the integumentary system, including skin, hair, and nails, through inspection and palpation. Understand how to evaluate nail health based on color, shape abnormalities like spoon shape or clubbing, and texture variations. Explore skin assessment criteria,
0 views • 21 slides
Understanding Stoichiometry and The Mole Concept in Chemistry
Explore the fundamentals of stoichiometry and the mole concept in chemistry, including conversions between moles and particles, molar mass calculations, and gram mole conversions. Learn how to determine the number and kinds of atoms in chemical formulas and understand the significance of Avogadro's
0 views • 12 slides
Understanding Toxicity: Mole-Mass Conversions in Chemistry
Explore the toxic nature of arsenic compounds and learn how to convert between moles and mass in this chemistry lesson. Understand the relationships between mass, moles, and the number of particles in a substance, and practice converting moles to mass and vice versa. Work in pairs to delve into the
0 views • 10 slides
Understanding the Ideal Gas Law in Chemistry
Exploring the concept of the Ideal Gas Law, its derivation from the combined gas law, conversion of pressures, practical applications through problem-solving examples, and the significance of the gas constant (R) in calculations. Learn how to use the Ideal Gas Law formula (PV = nRT) to solve for var
1 views • 19 slides
Polymer Molecular Weight Exercise Analysis
This exercise involves calculating the number average and weight average molecular weights, as well as the polydispersity index (PDI) for a sample of polystyrene composed of fractions with different molecular weights. The analysis includes determining the number of moles in each fraction, calculatin
0 views • 7 slides
Understanding the Mole Concept in Chemistry
Explore the fundamentals of the mole concept in chemistry, including calculations involving molecular formulas, reagent masses, percentage yields, gas volumes, concentrations, and balanced chemical equations. Learn about the definition of a mole, calculating the number of moles, and converting mass
0 views • 23 slides
Understanding Moles in Chemistry
Matter is composed of various particles, and chemists use the concept of moles as a unit of measure to quantify the number of particles in a substance. One mole is equal to 6.02 x 10^23 representative particles of a substance, known as Avogadro's number. Moles are versatile and applicable to differe
0 views • 25 slides
Understanding Partial Molar Quantities and Chemical Potential
Exploring partial molar quantities and chemical potential in thermodynamics helps us understand how system variables change with composition alterations. Through concepts like partial molar volumes and Gibbs free energy, we can delve into the intricate dynamics of thermodynamic systems and their beh
0 views • 23 slides
Understanding Molar Mass and Conversions in Chemistry
Explore the concept of molar mass, converting between grams and moles, determining mass and moles of elements, and calculating the number of atoms in samples. Practice exercises help reinforce learning in this comprehensive chemistry topic.
0 views • 15 slides
Understanding Molar Mass and Conversions in Chemistry
Explore the concept of molar mass, moles of atoms, and conversions between mass and moles in chemistry through an engaging lesson on toxins, stoichiometry, solution chemistry, and acids and bases. Learn how to calculate molar mass, describe the magnitude of a mole of a substance, and conduct simple
0 views • 10 slides
Understanding Gas Moles and Volumes in Steel Story Lesson 2
Dive into the world of moles in gases and solutions in Steel Story Lesson 2. Learn about molar volumes, gas calculations, solution concentrations, and more. Explore the essential concepts and formulas to enhance your understanding of chemical equations and volumes in various conditions.
0 views • 26 slides
Understanding Concentration and Dilution of Solutions by Dr. Laila Al-Harbi
Dr. Laila Al-Harbi explains the concept of concentration of solutions, focusing on molarity and the calculation involved. The molar concentration of solutions is determined by the amount of solute in a given volume of solvent, expressed as moles of solute per liter of solution. Additionally, the pro
0 views • 15 slides
Understanding Solution Concentration: Lesson in Chemistry
Explore the concept of solution concentration through an engaging experiment involving gummy bears in sugar and water solutions. Learn about key terms such as solution, solute, solvent, saturated solution, concentration, and molarity. Discover how molecules move based on concentration gradients and
0 views • 10 slides