Chemical equilibrium
Chemical equilibrium is a state where reactants and products reach a balance in a reaction. Learn about reversible and irreversible reactions, equilibrium constants, and how they affect chemical analysis. Discover the accuracy and applications of volumetric analysis, including titration methods like
3 views • 13 slides
Understanding Equilibrium in Rigid Bodies
Explore the concept of equilibrium in rigid bodies through problem-solving scenarios involving forces, moments, and tensions. Learn how to ensure balance and stability in resting bodies by analyzing forces and moments. Diagrams and step-by-step calculations help in understanding the physics behind e
0 views • 6 slides
Understanding Frictional Forces and Equilibrium Principles in Physics
Explore the concepts of frictional forces and equilibrium in physics with a focus on conditions for forces acting on a body associated with friction. Learn about limiting frictional force, coefficient of friction, and the equilibrium of a body on horizontal and inclined planes. Delve into scenarios
2 views • 9 slides
Understanding Equilibrium for Moving Objects
Objects can be in static equilibrium when at rest or dynamic equilibrium when moving at a constant speed. Equilibrium is maintained when there is no net force to change the state of motion. This equilibrium is possible when forces either cancel out or there is no force acting on the object. Friction
0 views • 8 slides
Understanding Mechanical Equilibrium in Physics
Mechanical equilibrium is a critical concept in physics where an object experiences no physical changes when the net force on it is zero. This state is described mathematically by the equilibrium rule, stating that the sum of forces acting on an object is equal to zero. In scenarios like a gymnast h
0 views • 10 slides
Understanding CGE and DSGE Models: A Comparative Analysis
Explore the similarities between Computable General Equilibrium (CGE) models and Dynamic Stochastic General Equilibrium (DSGE) models, their equilibrium concepts, and the use of descriptive equilibria in empirical modeling. Learn how CGE and DSGE models simulate the operation of commodity and factor
4 views • 15 slides
Understanding Chemical Equilibrium in Reversible Reactions and Laws
Chemical equilibrium in reversible reactions involves the balance between forward and backward reactions, as governed by laws like the law of mass action and the law of chemical equilibrium. These laws help in understanding the rates of reactions, equilibrium constants, and the relationship between
1 views • 12 slides
Understanding Dynamic Equilibrium in Chemical Reactions
Explanation of reversible reactions, dynamic equilibrium, and the characteristics of equilibrium in chemical systems. Covers the concept of reversible reactions, dynamic equilibrium, rules for dynamic equilibrium, and examples to illustrate these concepts visually.
0 views • 54 slides
Understanding Le Chatelier's Principle in Chemical Equilibrium
Le Chatelier's Principle states that when a system at equilibrium is disturbed by changes in concentration, temperature, or pressure, the equilibrium shifts to counteract the change. This principle can be applied to predict the direction of equilibrium when changes occur. Changes in concentration, p
0 views • 10 slides
Understanding Reaction Isotherms and Equilibrium Constants
Explore reaction isotherms and equilibrium constants through Vant Hoff's and Gibbs free energy equations. Learn about the relationship between Gibbs free energy, equilibrium constant, and temperature dependence. Discover how these concepts are applied in determining the direction of chemical reactio
1 views • 18 slides
Understanding Ionic Bonding and Lattice Energy
Explore the world of ionic bonding through images and explanations. Learn how electrons are transferred to form ions, the arrangement of ions in a crystal lattice, and the concept of lattice energy in ionic compounds. Discover the formation of formula units, examples of bond pairs, and the significa
1 views • 18 slides
Equilibrium of Rigid Bodies: Moments, Couples, and Forces
The topic covers the equilibrium of rigid bodies with a focus on moments, couples, and forces. It discusses concepts such as moments of a force, Varignon's theorem, types of supports, and equilibrium in two and three dimensions. The equilibrium in two-force members and three-force members is explain
0 views • 23 slides
Equilibrium Analysis in Cournot Competition Model
The analysis focuses on a Cournot competition model between two identical countries with one producer each. The model includes linear cost functions, demand functions, revenue calculations, profit maximization conditions, and equilibrium determination in both domestic and foreign markets. By solving
0 views • 7 slides
Chemical Bonding and Compound Formulas: Understanding Ionic vs. Covalent Bonds
Explore the differences between ionic and covalent bonds, learn about ionic compounds held by electromagnetic attractions, understand molecular compounds with shared electrons, and grasp the naming conventions for ions. Discover how molecular formulas and formula units represent atoms in compounds.
0 views • 56 slides
Understanding Ship Stability: Centre of Gravity and Metacentre
Exploring the concepts of transverse statical stability, centre of gravity, centre of buoyancy, metacentre, stable equilibrium, unstable equilibrium, and neutral equilibrium in ship stability. The relationship between these key points determines a ship's stability and ability to maintain a steady po
0 views • 8 slides
Understanding Price Determination in Livestock Economics and Marketing
Price determination under perfect competition involves the interaction of demand and supply curves to reach equilibrium, where the quantity demanded and supplied are balanced at an equilibrium price. In perfect competition, price is determined at the point where demand and supply intersect. Demand v
0 views • 16 slides
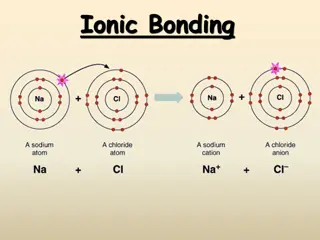
Understanding Ions, Ionic Bonds, and Ionic Compounds
Ions are charged particles formed by gaining or losing electrons, leading to the formation of ionic bonds between positively charged cations and negatively charged anions. The octet rule guides electron configurations, and the periodic table helps predict ion formation. Ionic bonding involves electr
0 views • 13 slides
Understanding Consumer Equilibrium in Economics
Consumer equilibrium refers to the point where a consumer maximizes satisfaction by spending income on commodities. In single commodity case, equilibrium is achieved when marginal utility equals price. For two commodities, equilibrium is reached when the ratio of marginal utility to price is equal.
0 views • 7 slides
Understanding Free Energy, Reaction Quotient, and Equilibrium Constant
This educational material delves into the concepts of free energy, reaction quotients, and equilibrium constants in chemical systems. It explains how to determine the direction of a reaction based on Q and K values, elucidates the role of Gibbs free energy in determining spontaneity, and provides ca
0 views • 10 slides
Understanding Chemical Equilibrium in Reversible Reactions
Chemical equilibrium occurs when the concentrations of reactants and products remain constant over time in a reversible reaction. Reaction rate is proportional to concentration, and equilibrium is reached when the rate of formation equals the rate of consumption in both directions. Reversible reacti
0 views • 25 slides
Understanding Cardiac Action Potential: Ionic Basis and Excitability in Cells
Explore the complex processes underlying cardiac action potential, from ionic equilibrium to resting membrane potential and excitability in cardiac cells. Learn about the critical thresholds, equilibrium potentials, and gradients that regulate the electrical activity of the heart. Discover the intri
0 views • 68 slides
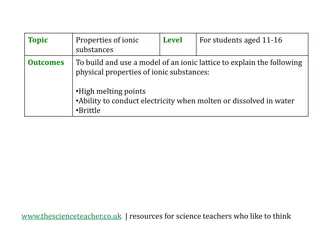
Exploring Physical Properties of Ionic Substances through Model Building
Engage students aged 11-16 in an interactive activity to understand the physical properties of ionic substances such as high melting points, ability to conduct electricity, and brittleness. By building a model of an ionic lattice for sodium chloride and explaining how the structure relates to these
0 views • 5 slides
Understanding Chemical Bonding and Atomic Properties
Explore the formation of ionic and covalent bonds, electron configurations of ions, and molecular geometry. Learn about ionic compound formation, atomic properties like effective nuclear charge, atomic size, ionization energy, and electron affinity. Discover the essential concepts of cations formati
1 views • 73 slides
Understanding Electrolytes and Ionic Equilibrium in Chemistry
Electrolytes play a crucial role in conducting electricity through ionization in aqueous solutions, with examples of strong and weak electrolytes explained. The concept of ionic equilibrium, degree of dissociation, and distinction between non-electrolytes are also covered in this comprehensive overv
0 views • 21 slides
Insights into Persuasion and Equilibrium in Multidimensional Cheap Talk
Explore the dynamics of multidimensional cheap talk, focusing on sender-receiver interactions, influential equilibrium, welfare rankings, and fragility to asymmetries. Lessons touch on bubbling equilibrium, influential equilibrium issues, welfare rankings preferences, and the impact of asymmetric pr
0 views • 20 slides
Understanding Equilibria in Populations: Hardy-Weinberg Principle
Exploring the concept of equilibria in populations, focusing on Hardy-Weinberg principles and its implications. The discussion covers allele distributions, genotype frequencies, maintenance of equilibrium across generations, and scenarios where equilibrium may be violated. Key points include basic p
0 views • 58 slides
Understanding Ionic and Molecular Compounds in Chemistry
Discover the fundamental concepts of ionic and molecular compounds in chemistry with insights into the nature of elements, formation of compounds, and properties of ions. Explore the differences between ionic and covalent bonds, positive and negative ions, as well as examples of common everyday comp
0 views • 54 slides
Understanding Groundwater Sustainability and Equilibrium Dynamics
This content delves into the concepts of groundwater sustainability, equilibrium, and capture in relation to groundwater management. It explores transitional storage, groundwater mining, and debunks the water budget myth. The images and explanations illustrate how pumping affects aquifers, the evolu
0 views • 11 slides
Understanding Ionic Bonding and Octet Rule in Chemistry
Understanding the concept of ionic bonding and octet rule in chemistry is essential for grasping how atoms combine to form molecules through sharing or exchanging electrons. This process involves the formation of positive and negative ions held together by electrostatic attraction, leading to the cr
0 views • 12 slides
Understanding Ionic Bonding and Lattice Energy in Chemistry
Chemical bonds play a crucial role in holding atoms together in molecules. This course explores the concept of chemical bonding, focusing on ionic bonds and lattice energy. Topics covered include the different types of chemical bonds, such as electrovalent and coordinate bonds, as well as the models
0 views • 22 slides
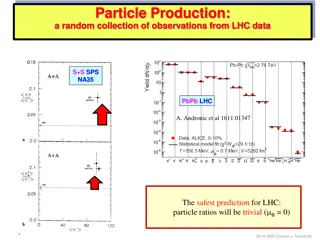
Insights into Particle Ratios and Equilibrium Dynamics at LHC
Collection of observations from LHC data regarding particle ratios and the successful Thermal Model. Discusses the concept of equilibrium, onset of equilibrium, relationship to QGP phase, and potential solutions through out-of-equilibrium studies. Also delves into size/volume dependence, strangeness
0 views • 11 slides
Understanding Homogenous Chemical Equilibrium
Homogenous chemical equilibrium occurs when reactants and products are in the same phase. This equilibrium remains independent of the volume of the reaction mixture. The concept is illustrated through the example of the Hydrogen-Iodide system and a generic reaction A + B --> 2C. Partial pressure pla
0 views • 56 slides
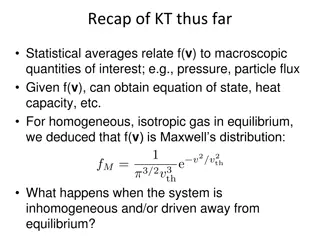
Insights into Non-equilibrium Kinetic Theory: Inhomogeneous Systems
Statistical averages in kinetic theory connect distribution functions to macroscopic properties like pressure and particle flux. When systems are inhomogeneous or away from equilibrium, local equilibrium breaks down, leading to slow relaxation processes towards global equilibrium. The evolution of p
0 views • 12 slides
Understanding Equilibriums in Physics
Equilibrium in physics refers to the state of a body where there is no change in translational or rotational motion. This state can be classified into static equilibrium (when total force and torque are zero) and dynamic equilibrium (when a body is in uniform motion with zero resultant force and tor
0 views • 9 slides
Understanding Market Equilibrium
Market equilibrium is achieved when the quantity demanded equals the quantity supplied at a specific price, ensuring a balance in the marketplace. Demand and supply schedules play a crucial role in determining market equilibrium, with excess supply or demand occurring when prices deviate from the eq
0 views • 12 slides
Understanding Game Abstraction and Equilibrium
Extensive-Form Game Abstraction with Bounds delves into the complexities of game abstraction, exploring theoretical guarantees, algorithmic challenges, and equilibrium-finding processes. The difficulty of game abstraction is examined, highlighting issues such as pathologies and the struggle to optim
0 views • 22 slides
Understanding Ionic Bonding and Naming in Chemistry
Exploring the fundamentals of ionic bonding, naming conventions, and the Octet Rule in chemistry. Learn about Lewis structures, formation of ionic compounds, and the role of valence electrons in determining chemical properties. Discover how elements gain or lose electrons to achieve a full outer ene
0 views • 24 slides
Understanding Chemical Equilibrium in Chemistry
Exploring the concept of chemical equilibrium in chemistry, where reactions can occur in both forward and reverse directions to an appreciable extent. Learn about basic equilibrium principles, equilibrium problems, manipulating reactions, and common example problems in nomenclature. Understand how r
0 views • 24 slides
Understanding Bonding in Ionic, Covalent, and Metallic Compounds
Explore the concepts of ionic, covalent, and metallic bonding through an investigation conducted by Vanderbilt Student Volunteers for Science. Learn about the different types of bonding, properties of ionic and molecular compounds, and the conductivity of metals. Discover the importance of determini
0 views • 15 slides
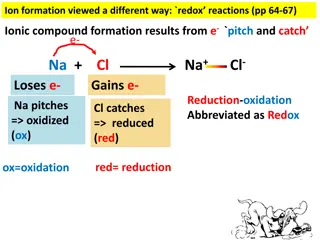
Understanding Redox Reactions and Ionic Compound Formation
Explore the concept of redox reactions through the process of ion formation, where elements lose or gain electrons to create ionic compounds. Learn about oxidation (ox) and reduction (red) in chemical reactions, and how to identify which elements lose or gain electrons based on charge changes. Disco
0 views • 14 slides