Types of groups and reactions
This information discusses electron-donating groups (EDGs) and electron-withdrawing groups (EWGs), their effects on molecule reactivity, examples of each group, nucleophiles, and electrophiles. EDGs increase electron density, making nucleophiles stronger, while EWGs decrease electron density, making
0 views • 14 slides
Crystal Field Theory in Transition Metal Complexes
Crystal Field Theory (CFT) explains the colors and magnetic properties of transition metal complexes. It focuses on the energy changes in d-orbitals of metal ions caused by surrounding ligands. This theory, developed in 1929, provides insights into the bonding interactions in complex compounds. The
10 views • 44 slides
Advanced Techniques in Materials Science: Transmission Electron Microscopy
Explore the advanced techniques used in materials science, focusing on Transmission Electron Microscopy (TEM). Learn about the challenges with optical microscopes, the principles of TEM imaging, and the application of scattering theory in electron microscopy. Discover how TEM offers higher resolutio
3 views • 19 slides
Understanding Ionic and Metallic Bonding in Chemistry
Explore the concepts of ions, electron dot structures, the octet rule, cations, and anions in Chapter 7. Learn how elements achieve stability through electron configurations, and practice writing electron dot structures and naming ions. Understand the differences between cations and anions and how t
1 views • 52 slides
Advanced Microbunched Electron Cooling for EIC Design Overview
Microbunched electron cooling is a cutting-edge technique proposed for the Electron-Ion Collider (EIC) design, aimed at enhancing beam properties through coherent electron interactions. The concept utilizes Coherent Electron Cooling (CeC) and broad-band amplification in the form of Micro-bunched Ele
1 views • 16 slides
Exploring Quantum Theory and the Atom: Electrons in Atoms and the Periodic Table
Delve into the fascinating world of quantum theory and the atom in Chapter 9, where we compare Bohr's model with the quantum mechanical model. Understand de Broglie's wave-particle duality and Heisenberg's uncertainty principle's impact on our current electron view. Discover the relationships among
0 views • 31 slides
Understanding Hybridization in Organic Chemistry
Delve into the complexities of the Lewis octet model and the insights provided by Linus Pauling's localized valence bond hybridization model to explain bond shapes in molecules, reactivity trends, and electron distribution in double and triple bonds. Discover how hybridization transforms atomic orbi
0 views • 22 slides
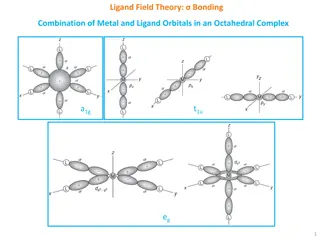
Understanding Ligand Field Theory in Octahedral Complexes
Ligand Field Theory explains the bonding interactions between metal and ligand orbitals in octahedral complexes. This theory involves the combination of metal and ligand orbitals to form molecular orbitals, leading to specific electronic configurations. The overlap of metal and ligand group orbitals
1 views • 10 slides
Understanding Crystal Field Theory in Chemistry
Crystal Field Theory (CFT) explains how electron orbital degeneracies, particularly d or f orbitals, are affected by a static electric field generated by neighboring anions. In CFT, the metal ion is considered positive while ligands are negative charges, leading to attractive and repulsive forces af
0 views • 13 slides
Understanding Electron Configurations and Atom Properties
Explore topics including electron configuration, full shells, atomic numbers, and properties of elements like Ytterbium, Bromine, Mercury, Magnesium, and Europium. Learn about isotopes, ions, and orbital electron distribution in atoms like Europium and Nitrogen, as well as practice completing electr
1 views • 10 slides
Understanding Cathode Ray Tubes (CRT) in Oscilloscopes
Cathode Ray Tubes (CRTs) are key components in oscilloscopes, modulating and accelerating electron beams to create images of electrical waveforms, radar targets, and more. Unlike TVs, CRTs in oscilloscopes use electrostatic deflection for precise beam control. The electron gun assembly consists of a
0 views • 18 slides
Exploring the Free Electron and Nearly Free Electron Models in Solid State Physics
The Free Electron Model postulates that electrons in metals move freely without interacting with crystal ions, yielding insights on conductivities. Developed by Arnold Sommerfeld, it combines the Drude model with quantum mechanics. Conversely, the Nearly Free Electron Model leans on quantum mechanic
0 views • 22 slides
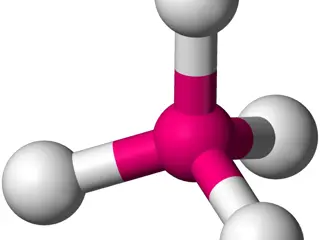
Understanding Covalent Bonds and Molecular Structure in Organic Chemistry
The neutral collection of atoms in molecules held together by covalent bonds is crucial in organic chemistry. Various structures like Lewis and Kekulé help represent bond formations. The concept of hybridization explains how carbon forms tetrahedral bonds in molecules like methane. SP3 hybrid orbit
0 views • 4 slides
Understanding Different Types of Chemical Bonds
Metallic bonds involve atoms giving up valence electrons to form an electron sea, covalent bonds entail electron sharing to fill outer orbitals, ionic bonds form when atoms with different electronegativities attract, Van der Waals bonds include London forces between atoms, and hydrogen bonds occur i
0 views • 6 slides
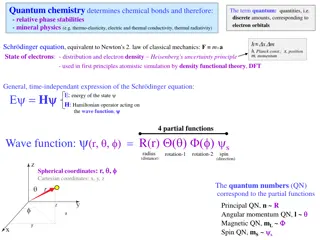
Understanding Quantum Chemistry and Electron Orbitals
Quantum chemistry plays a key role in determining chemical bonds, phase stabilities, and mineral physics through the study of electron orbitals, quantum numbers, and energy levels. This involves concepts such as the Schrödinger equation, quantum quantities, and the uncertainty principle. The arrang
0 views • 31 slides
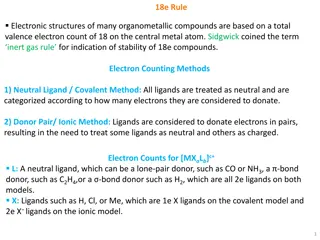
Understanding the 18-Electron Rule in Transition Metal Organometallic Compounds
The 18-electron rule governs the stability of transition metal organometallic compounds by requiring the sum of metal d electrons and ligand-supplied electrons to be 18. This rule highlights the importance of electron count and ligand characteristics in forming stable complexes. Key concepts include
0 views • 15 slides
Understanding Atomic Orbitals and Electron Arrangement
Learn about the properties of atomic orbitals and how they determine the distance from the nucleus and the shape of orbitals. Explore the main energy levels, sublevels, and the arrangement of electrons following the Aufbau Principle within an atom.
4 views • 28 slides
Understanding Alkynes in Organic Chemistry
Alkynes are unsaturated hydrocarbons with at least one triple bond, following a molecular formula of CnH2n-2. This group of compounds is discussed in Chapter three, covering topics like structure, hybridization, common naming, physical properties, preparation, and reactions. The sp hybridization of
1 views • 20 slides
Understanding Density Functional Theory in Chemistry
Density Functional Theory (DFT) plays a crucial role in chemistry by uniquely determining molecular properties based on electron density. The Hohenberg-Kohn Theorem establishes the foundation, with the goal of finding an exact energy functional expressed in terms of density. Various concepts like th
0 views • 19 slides
Accelerator Technology R&D Targets and Sources Overview
The SnowMass2021 Accelerator Frontier AF7 focuses on Accelerator Technology R&D, exploring targets and sources such as high brightness electron sources, muon sources, and high intensity ion sources. The community planning meeting discussed various Letter of Interest submissions outlining innovative
0 views • 7 slides
Understanding Electron Configuration and Quantum Numbers in Chemistry
Explore the concept of electron configurations, quantum numbers, and orbital filling rules in chemistry. Discover the principles governing the arrangement of electrons in atoms, including the Aufbau Principle, Pauli Exclusion Principle, and Hund's Rule. Gain insight into orbital energy levels and th
0 views • 18 slides
Crystal Field Theory and Color Exhibited by Coordination Compounds
Crystal Field Theory (CFT) explains the colors exhibited by coordination compounds based on the absorption of light and electron transitions in d-orbitals. The theory describes how ligands interact with transition metal ions, causing the d-orbitals to split in energy levels. This split results in th
0 views • 30 slides
Understanding Electron Correlation and Basis Sets in Molecular Calculations
Polarized basis sets describe the electron density polarization in atoms and molecules to improve accuracy in computed geometries and frequencies. Diffuse basis sets are recommended for calculating electron and proton affinities. Electron correlations account for electron interactions in molecular c
0 views • 8 slides
Understanding Electron-Phonon Interactions in Iron-Based Superconductors
This discussion explores the effects of electron-phonon interactions on orbital fluctuations in iron-based superconductors. Topics covered include ab initio downfolding for electron-phonon coupled systems, evaluation methods such as Constrained Random Phase Approximation (cRPA), Constrained Density-
0 views • 12 slides
Understanding Microwave Tubes and Klystron Technology
Microwave tubes play a crucial role in high-frequency applications due to their efficiency and operating principles. Conventional tubes face limitations beyond 100MHz, while efficient microwave tubes utilize electron velocity modulation for power conversion. Klystron tubes, such as Reflex Klystron,
4 views • 19 slides
Ion Beam Intensity Enhancement Through Electron Heating in Collider Experiments
The study discusses electron heating of ions in collider experiments at the Collider V. ParkhomchukBINP facility in Novosibirsk. It explores the effects of electron cooling on ion beams, ion beam oscillations, losses, and ion beam intensity enhancement. Various factors such as ion charge, classical
0 views • 9 slides
Understanding the 18e Rule in Organometallic Compounds
The 18e rule dictates the electronic structures of many organometallic compounds, emphasizing a total valence electron count of 18 on the central metal atom for stability. Electron counting methods like the Covalent and Ionic models assist in determining the electron distribution among ligands. The
0 views • 8 slides
Understanding Electron Configurations and the Periodic Table in Chemistry
Explore the world of electron configurations in atoms, subshells, and electron arrangement using the periodic table. Learn about the organization of electrons in subshells, different ways to represent electron arrangements, and how to determine electron configurations based on the periodic table. Di
0 views • 12 slides
Understanding Atomic Orbitals: Counting, Subshells, Energies, and Electrons
Learn about the basics of atomic orbitals, including the counting of orbitals in shells and subshells, the distribution of electrons in different energy levels, and the symmetrical nature of orbital labeling. Dive into the rules governing electron placement based on quantum mechanics and explore the
0 views • 6 slides
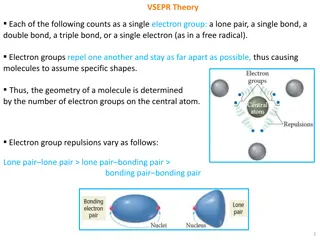
Understanding VSEPR Theory for Molecular Geometry
VSEPR theory explains how the arrangement of electron groups around a central atom determines the shape of molecules based on the repulsions between different types of electron groups. The geometry of a molecule is influenced by factors such as lone pairs, single, double, or triple bonds, and their
0 views • 16 slides
Understanding Microscopes: Light vs. Electron Microscopes
Learn about the differences between light microscopes (LM) and electron microscopes (EM), including their magnification power, resolving power, and key parts. Explore the types of electron microscopes such as Transmission Electron Microscope (TEM) and Scanning Electron Microscope (SEM) for advanced
0 views • 8 slides
Understanding Electron Microscopy: A Comprehensive Overview
Electron microscopy (EM) is a powerful technique used in biomedical research to visualize detailed structures of various specimens at high resolution. The process involves an electron gun, electromagnetic lenses, specimen holder, and imaging systems. There are two main types of electron microscopes:
0 views • 12 slides
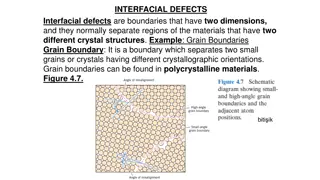
Understanding Interfacial Defects and Microscopy in Materials Science
Interfacial defects are boundaries separating regions with different crystal structures, like grain boundaries in polycrystalline materials. Microscopic examination distinguishes between macroscopic and microscopic dimensions, analyzed through optical and electron microscopy methods. Electron micros
0 views • 9 slides
Understanding Scanning Electron Microscopes (SEM) and Electron Sources
Scanning Electron Microscopes (SEMs) utilize focused electron beams to produce high-resolution images by interacting with a sample's electrons. The electron source, such as the electron gun, plays a crucial role in forming fine electron beams for imaging purposes. Different types of electron sources
0 views • 12 slides
Quantum Interactions: Electrons, Phonons, and Hubbard Interaction
Exploring the complexities of electron-electron and electron-phonon interactions, nonequilibrium Green's functions, Hartree-Fock method, Coulomb's law, quantum operator forms, Hubbard interaction, and electron-phonon interactions from first principles. The interactions delve into the behavior of cha
0 views • 20 slides
Design of a 10 MeV Beamline for E-beam Irradiation at UITF Wastewater Facility
This paper discusses the design of a 10 MeV beamline at the Upgraded Injector Test Facility for electron beam irradiation, focusing on wastewater treatment for environmental restoration. The use of electron beam irradiation is highlighted as an effective method to remove pollutants like 1,4 dioxane
0 views • 9 slides
Advancements in Machine Learning for Electron Density Prediction
Electron density is crucial for understanding atomic bonding. This research project explores using machine learning, specifically a Unet architecture, to predict electron density in a Lithium-Oxygen-Lithium system. The data set was generated by varying the positions of Lithium atoms and calculating
0 views • 8 slides
Advancing Electron Microscopy in Life Sciences through UEM Feasibility Demonstration
Demonstration project of the feasibility of a sub-nanometer, picosecond electron microscope for life sciences applications. The goal is to image biological cells with resolution below 200nm using a proof-of-concept system integrated with existing UED setup. The project builds on previous successes i
0 views • 14 slides
Fundamentals of Electron Beam Ion Sources for Ionization: A Comprehensive Overview
Delve into the intricacies of Electron Beam Ion Sources (EBIS) and Electron Beam Ion Traps (EBIT) with a focus on their historical development, key operating principles, and main concepts. Explore the production of high charge states for accelerator applications, electron beam confinement, ionizatio
0 views • 10 slides
Understanding Microbial Physiology: The Electron-NADP Reduction Pathway
Dr. P. N. Jadhav presents the process where electrons ultimately reduce NADP+ through the enzyme ferredoxin-NADP+ reductase (FNR) in microbial physiology. This four-electron process involves oxidation of water, electron passage through a Q-cycle, generation of a transmembrane proton gradient, and AT
0 views • 29 slides