Understanding Atomic Orbitals and Electron Arrangement
Learn about the properties of atomic orbitals and how they determine the distance from the nucleus and the shape of orbitals. Explore the main energy levels, sublevels, and the arrangement of electrons following the Aufbau Principle within an atom.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
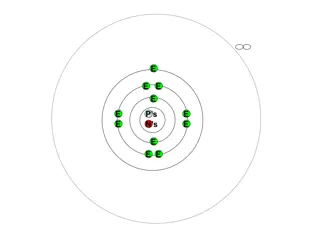
Number that specifies the properties of the atomic orbitals Tells us the distance from the nucleus and the shape of the orbital
Main level or shell These are the Bohr energy levels n = 1, n = 2, n = 3 As n increases, the distance from the nucleus increases
Each main level is divided into sublevels Four types of sublevels s p d f
Each sublevel is made of orbitals Every orbital can hold 2 electrons
s 1 orbital 2 electrons p 3 orbitals 6 electrons d 5 orbitals 10 electrons f 7 orbitals 14 electrons
One spherical shaped orbital
Three dumbbell shaped One dumbbell in each axis
Main Level Main Level Sublevels Sublevels Number of Sublevels Number of Sublevels Electrons in sublevels Electrons in sublevels Total Electrons in Main Level Total Electrons in Main Level 1 s 1 2 2 2 s p 1 3 2 6 8 3 s p d 1 3 5 2 6 18 10 4 s p d f 1 3 5 7 2 6 32 10 14
Arrangement of electrons in an atom Aufbau Principle electrons fill into an atom starting with the lowest energy levels
Way which the electrons rotate on their axis Pauli Exclusion Principle in order for two electrons to occupy the same orbital, they must have opposite spin
File:Orbital diagram nitrogen.svg Hund s Rule electrons occupy orbitals singly first before doubling up
Write the configuration for each of the below C S Br Na Cl Kr
Electrons in the last main energy level These are the electrons farthest out on the atom These will interact with other atoms These are the electrons involved in chemical reactions There are a maximum of 8 valence electrons
Write configuration and count electrons in last main energy level Examples: Find valence electrons for C Na P Fe Ar
Atoms will give up, accept, or share electrons in order to achieve a filled valence shell (8 valence electrons)
Metals become more reactive (more metallic in character) as you go down a group Most metallic elements bottom left corner of the periodic table Least metallic, top right corner
Energy required to remove the most loosely held electron from an atom The greater the ionization energy, the more strongly the atom holds onto its electrons M + energy M+ + e Ionization energy increases as moving across a period Ionization energy decreases as moving down a group
Half of the distance between two adjacent nuclei Radius decreases across a period (atoms hold the electrons tightly in) Radius increases down a group