Types of groups and reactions
This information discusses electron-donating groups (EDGs) and electron-withdrawing groups (EWGs), their effects on molecule reactivity, examples of each group, nucleophiles, and electrophiles. EDGs increase electron density, making nucleophiles stronger, while EWGs decrease electron density, making
0 views • 14 slides
Organometallic Chemistry (CHEM 42 1)
Organometallic chemistry delves into compounds with carbon-metal bonds, merging concepts from inorganic and organic chemistry. The field encompasses diverse compounds like ferrocene and tris(triphenylphosphine)rhodium carbonyl hydride, with nomenclature based on naming organic groups and adding meta
1 views • 13 slides
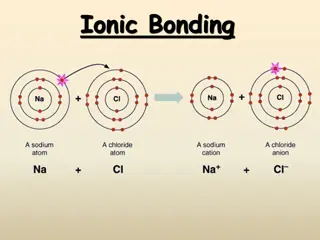
Ionic and Covalent Bonding in Chemistry
Ionic bonding involves the transfer of electrons between a metal and a non-metal to form a giant lattice structure, like in sodium chloride and lithium oxide. Covalent bonding, on the other hand, occurs between non-metals, resulting in giant covalent structures or simple molecules. Examples such as
4 views • 79 slides
Covalent Bonding in Chemistry
Explore the concept of covalent bonding in chemistry, where atoms share electrons through orbital overlap to form stable molecules. Learn about why covalent bonds exist, how bond length affects the stability of a molecule, the model for covalent bonding, Lewis structures, and the characteristics of
0 views • 12 slides
Metallic Bonding and Giant Metallic Lattices
Metallic bonding involves the attraction of positive metal ions to delocalized electrons, forming giant metallic lattices. In this structure, positive metal ions occupy fixed positions while electrons move freely throughout. This bonding is different from covalent bonding as it is delocalized, leadi
1 views • 19 slides
Atomic Properties and Covalent Radii in Chemistry
Exploring the concept of atomic properties including the sizes of atoms and ions, the three common operational radius concepts (covalent, crystal, and van der Waals), and the calculation of covalent radius for homonuclear and heteronuclear diatomic molecules. This overview delves into the significan
1 views • 10 slides
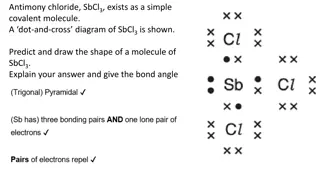
Chemical Bonding Concepts and Structures Explanation
Explore the concepts of chemical bonding through dot-and-cross diagrams for molecules like Antimony Chloride (SbCl3) and Boron Tribromide, along with explanations on ionic lattice structures, covalent bonds, and electrical conductivity in substances like Aluminium Fluoride (AlF3). Understand the sha
0 views • 9 slides
Drug Action Mechanisms and Receptor Targets
Pharmacodynamics involves studying the effects of drugs on biochemical and physiological levels, focusing on drug actions through receptor-mediated and non-receptor mechanisms. The interaction between drugs and targets like proteins and enzymes impacts cellular functions. Different binding forces, s
0 views • 9 slides
Dielectrics and Insulators in Electrical Engineering
Dielectric materials, also known as insulators, have tightly bound electrons with no free charges, characterized by a forbidden energy band gap of over 4 eV. Insulators, on the other hand, prevent electric current flow due to high resistivity and strong covalent bonds. Learn about the differences, d
1 views • 22 slides
Enzyme Catalysis and Active Site Role
Enzymes play a crucial role in catalyzing biochemical reactions by stabilizing transition states through their active sites. Different mechanisms like acid-base, covalent, metal, and electrostatic interactions are employed for stabilization. Acid-base catalysis involves acceleration without being co
1 views • 21 slides
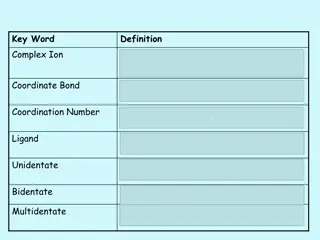
Complex Ions and Coordinate Bonds in Chemistry
Complex ions in chemistry are formed when transition metals or their ions bond with ligands through coordinate bonds. Ligands utilize their lone pairs of electrons to form dative covalent bonds with transition metals, determining the coordination number of the cation. Complex ions play a crucial rol
1 views • 29 slides
Chemical Groups and Macromolecules in Biological Processes
In biological processes, certain chemical groups play crucial roles in molecular functions. These functional groups, including hydroxyl, carbonyl, carboxyl, amino, sulfhydryl, phosphate, and methyl, are essential for the structure and function of biological molecules. Additionally, macromolecules, s
0 views • 9 slides
Covalent Bonds and Molecular Structure in Organic Chemistry
The neutral collection of atoms in molecules held together by covalent bonds is crucial in organic chemistry. Various structures like Lewis and Kekulé help represent bond formations. The concept of hybridization explains how carbon forms tetrahedral bonds in molecules like methane. SP3 hybrid orbit
0 views • 4 slides
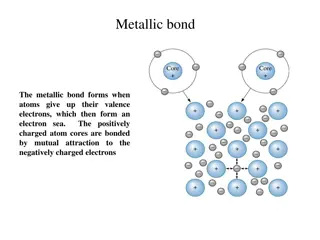
Different Types of Chemical Bonds
Metallic bonds involve atoms giving up valence electrons to form an electron sea, covalent bonds entail electron sharing to fill outer orbitals, ionic bonds form when atoms with different electronegativities attract, Van der Waals bonds include London forces between atoms, and hydrogen bonds occur i
0 views • 6 slides
Bonding in Chemistry
Delve into the world of chemical bonding through ionic, covalent, and metallic bonds. Explore how elements form bonds, from the attraction between sodium and chloride ions to the sharing of electrons in covalent bonds. Witness the formation of compounds like sodium chloride and magnesium oxide, unde
1 views • 12 slides
Chemical Bonding and Stability in Atoms
Explore the significance of chemical bonds in providing stability to atoms through ionic and covalent bonding mechanisms. Learn about valence electrons, types of bonds, and why atoms form bonds for enhanced stability.
0 views • 16 slides
Understand Molecular Structures with Lewis Dot Symbols
Explore the world of molecular structures with Lewis dot symbols in this chemistry unit. Learn about valence electrons, covalent bonding, and the HONC 1234 rule through engaging activities and discussions. Create accurate structural formulas and describe bonding in molecular substances. Get ready to
0 views • 13 slides
Molecular Imprinting in Artificial Antibodies
Molecular imprinting is a technique used to create synthetic antibodies with specific recognition sites, allowing for applications in chiral chromatography, immunoassays, sensor development, and more. Imprinted polymers offer advantages such as target-defined recognition sites and stability in vario
3 views • 15 slides
Chemical Bonding and Compound Formulas: Understanding Ionic vs. Covalent Bonds
Explore the differences between ionic and covalent bonds, learn about ionic compounds held by electromagnetic attractions, understand molecular compounds with shared electrons, and grasp the naming conventions for ions. Discover how molecular formulas and formula units represent atoms in compounds.
1 views • 56 slides
Chemistry: Naming Compounds and Writing Formulas
Understand compounds, chemical formulas, and how to write ionic formulas using the Swap 'n Drop Method. Learn about types of compounds - ionic, covalent, and acids, and practice writing formulas for various elements. Follow rules, naming flow charts, and partner activities to enhance your understand
1 views • 13 slides
Insights into Coordination Chemistry Elements and Complexes
Transition elements with d or f electrons possess unique properties, play crucial roles in biological processes, and form colorful complexes with ligands. Occurring widely in nature, these elements have varied oxidation states and coordination numbers. Werner's formulation sheds light on primary and
0 views • 41 slides
Interactions of Planar Organic Radicals: Stacking and Bonding
Examination of the stacking interactions and bonding in planar organic radicals reveals a variety of non-covalent and weak covalent interactions such as hydrogen bonding, halogen bonding, and pancake bonding. This study highlights the significance of multicentric two-electron bonding and explores th
0 views • 29 slides
Chemical Bonding: Valency, Formulas, and Reactions
Explore the world of chemical bonding with this unit covering valencies, chemical formulas, ionic vs. covalent bonds, and exothermic vs. endothermic reactions. Learn to predict element combinations, create molecular formulas, and differentiate between various bond types. Jigsaw diagrams demonstrate
1 views • 46 slides
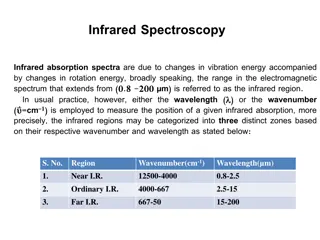
Infrared Spectroscopy: An Overview of Vibration Energy and Spectral Regions
Infrared spectroscopy involves analyzing absorption spectra resulting from changes in vibration and rotation energy in molecules. The infrared region spans from 0.8 to 200 μm, with distinct zones categorized based on wavenumber and wavelength. Group frequencies and fingerprint regions offer detaile
1 views • 26 slides
Chemistry Revision Mind Map Unit 1 Summary
Exploring Unit 1 of Chemistry revision, we delve into bonding of the first 20 elements, trends in the periodic table, structure and bonding concepts, and oxidation and reduction reactions. Topics covered include melting points, boiling points, covalent radius, ionization energy, types of bonding, in
0 views • 4 slides
Intermolecular Forces: Strength, Types, and Examples
Intermolecular forces are attractions between molecules, weaker than chemical bonds. They include London dispersion forces, dipole-dipole interactions, and hydrogen bonding. Strength varies, with covalent bonds being the strongest and London dispersion forces the weakest. Different types of intermol
0 views • 15 slides
Chemical Bonding and Atomic Properties
Explore the formation of ionic and covalent bonds, electron configurations of ions, and molecular geometry. Learn about ionic compound formation, atomic properties like effective nuclear charge, atomic size, ionization energy, and electron affinity. Discover the essential concepts of cations formati
1 views • 73 slides
Naming of Polyatomic Ions, Acids, and Covalent Compounds
Exploring the nomenclature of polyatomic ions, oxyanions, acids, and covalent compounds. Learn how to name compounds based on their composition, whether they contain oxygen, and the type of bond they form.
0 views • 16 slides
Ceramic Properties: Strength, Brittle Behavior, and More
Ceramics exhibit specific properties such as high compressive strength, brittleness due to mixed ionic-covalent bonding, low fracture toughness, poor electrical and thermal conduction (except for some types), and chemical insensitivity. While strong in compression, ceramics are brittle and lack duct
0 views • 13 slides
Ionic and Molecular Compounds in Chemistry
Discover the fundamental concepts of ionic and molecular compounds in chemistry with insights into the nature of elements, formation of compounds, and properties of ions. Explore the differences between ionic and covalent bonds, positive and negative ions, as well as examples of common everyday comp
0 views • 54 slides
Chemical Bonds and Molecular Geometry
Chemical bonds are the forces that hold atoms together, with valence electrons playing a crucial role. Ionic bonds involve complete electron transfer between metals and nonmetals, while covalent bonds see electrons being shared. Lewis dot diagrams help in visualizing the valence electrons of atoms,
0 views • 68 slides
Chemical Bonds: Covalent, Ionic, and Metallic
Explore the fascinating world of chemical bonds, including covalent bonds where atoms share electron pairs (e.g., water), ionic bonds where oppositely charged ions attract (e.g., sodium chloride), and metallic bonds formed between positively charged atoms sharing free electrons (e.g., copper wire).
0 views • 6 slides
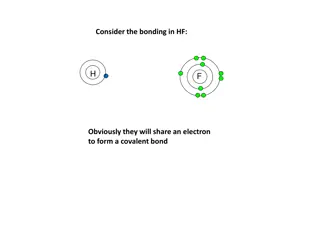
Bonding in HF Molecule
In HF bonding, hydrogen and fluorine share an electron to form a covalent bond. Fluorine, being more electronegative, attracts the bonding electrons more, resulting in a polar covalent bond. If hydrogen was less electronegative, the bonding electrons would shift further towards fluorine until an ion
0 views • 11 slides
Covalent Bonds and Nomenclature
Explore the concepts of covalent bonding, where electrons are shared between atoms, leading to the formation of stable molecules. Learn the nomenclature rules for naming covalent compounds using prefixes and root names. Discover the nature of diatomic molecules and the naming conventions for acids.
0 views • 42 slides
Ionic Bonding and Octet Rule in Chemistry
Understanding the concept of ionic bonding and octet rule in chemistry is essential for grasping how atoms combine to form molecules through sharing or exchanging electrons. This process involves the formation of positive and negative ions held together by electrostatic attraction, leading to the cr
0 views • 12 slides
Crystal Field Theory and Atomic Basis Sets
Explore the principles behind crystal field theory, including the concept of effective Hamiltonians to mimic covalent bonding in solids. Learn how to expand potentials on spherical harmonics and understand the selection rules and symmetry considerations in this theoretical framework.
0 views • 8 slides
Non-Covalent Pi-System Interactions in Molecular Structures
Non-covalent interactions play a crucial role in chemical selectivity and molecular recognition. This article discusses the significance of Pi-system interactions, including Pi-Pi and Cation-Pi interactions, in stabilizing molecular structures like DNA helices and G-quadruplexes. Insights into molec
0 views • 6 slides
Enzyme Regulation and Factors Affecting Enzyme Activity
Organisms carefully control enzyme production and activation as per varying needs and conditions within cells. Enzyme activity is influenced by factors such as pH, temperature, regulatory molecules, cofactors, compartmentalization, covalent modification, and feedback inhibition. Enzymes can be regul
0 views • 20 slides
Electronic Effects and Stability of Reaction Intermediates
Explore the distribution of electron density in molecules, inductive effect, mesomeric effect, resonance, and the relative stability of reaction intermediates. Learn about induction and polar covalent bonds, electron-withdrawing and electron-donating groups, and the impact of formal charges on molec
0 views • 13 slides
Bonding in Ionic, Covalent, and Metallic Compounds
Explore the concepts of ionic, covalent, and metallic bonding through an investigation conducted by Vanderbilt Student Volunteers for Science. Learn about the different types of bonding, properties of ionic and molecular compounds, and the conductivity of metals. Discover the importance of determini
1 views • 15 slides