Compliance with Audit Documentation
Understand the significance of audit documentation in proving compliance with audit requirements, ensuring quality audit work, and providing evidence for audit conclusions. Learn about the purpose, requirements, and definitions of audit documentation as outlined in auditing standards. Proper documen
6 views • 40 slides
Active Tuberculosis Drug Safety Monitoring and Management Training Package 2023
This training package focuses on the safety monitoring and management of active tuberculosis drugs, specifically emphasizing causality assessment in determining adverse reactions. Participants will learn about assessing the likelihood of TB medicines causing adverse events, attributing relationships
3 views • 17 slides
Overview of Serious Adverse Reactions and Transfusion Events
This data compilation covers the reporting trends, breakdown of reports, components issued, and specific types of adverse transfusion reactions experienced within the National Healthcare Organization (NHO) from 2019 to 2022. The information includes statistics on Serious Adverse Events (SAE), Seriou
2 views • 46 slides
Understanding Adverse Events Following Immunization (AEFI)
Adverse Events Following Immunization (AEFI) are medical incidents that occur after immunization, potentially caused by the vaccine, leading to unfavorable symptoms. Pharmacovigilance plays a crucial role in detecting, assessing, and preventing these events. AEFI can impact immunization programs at
4 views • 40 slides
NSFAS Annual Report 2021/22 - Adverse Audit Findings and Resolutions Update
Significant delays affected the compilation of NSFAS Annual Financial Statements for 2021/22, leading to an adverse audit opinion due to material misstatements and limitations in various financial areas. The report highlights issues with amounts owed, receivables, bursaries, cash flows, irregular ex
0 views • 38 slides
Understanding Adverse Events in Clinical Trials
This presentation covers the identification, recording, and reporting of adverse events in clinical trials conducted by University of Edinburgh and/or NHS Lothian. It includes definitions, examples of adverse event recording, SAE form completion, and common mistakes. AEs can range from minor issues
0 views • 35 slides
Understanding the Single Audit Process for Federal Grants
The single audit process is a requirement for non-federal entities receiving over $750,000 in Federal awards, ensuring compliance with program requirements and Uniform Guidance. This audit involves examining financial statements and ensuring proper fund utilization. Auditee responsibilities and key
0 views • 36 slides
Audit of Ward-based IT Infrastructure at University Hospital of Limerick
In response to a cyber-attack on the HSE in 2021, an audit was conducted at University Hospital of Limerick to assess the accessibility of key healthcare-related computer programs. The audit revealed issues such as JavaScript errors, missing plug-ins, outdated software, and internet access issues. I
0 views • 4 slides
Association of Renal and Cerebral Near-Infrared Spectroscopy with Adverse Outcomes in Single Ventricle Patients after Stage I Palliation
Study investigates the association between renal and cerebral near-infrared spectroscopy (NIRS) values and low cardiac output syndrome in single ventricle patients post Stage I palliation. Data from infants who underwent surgery between 2010-2019 is analyzed to determine correlations with adverse ou
1 views • 13 slides
Internal Audit Process & Audit Committees Overview
Explore the functions and responsibilities of internal audit processes and audit committees in the context of school boards in Ontario. Covering topics such as governance, risk management, compliance, and internal controls, the content delves into the structure, mandate, and activities of internal a
2 views • 34 slides
New Risk Management and Internal Audit Framework for Local Councils in NSW
This framework outlines the importance of audit, risk, and improvement committees (ARIC), internal audit (IA), and risk management (RM) in local councils in NSW under the Local Government Act 1993. It defines key terms, such as Audit Committee, Internal Audit, Risk Management, and the three lines of
1 views • 31 slides
National Audit Results for Severe Sepsis and Septic Shock 2016/17
This presentation outlines the national results of a clinical audit conducted in 2016/17 regarding the management of severe sepsis and septic shock in Emergency Departments (EDs). The audit objectives include benchmarking current performance, facilitating national and peer comparisons, identifying a
0 views • 25 slides
Impact of Data Analytics and Consulting Activities on Internal Audit Quality
This research examines how the use of data analytics and consulting activities affect perceived internal audit quality. The study investigates the relationship between these factors and top management's perception of internal audit quality. Through online scenario-based experiments with middle and t
2 views • 11 slides
National Clinical Audit 2016/17: Consultant Sign-Off Summary
This presentation displays the national results of the 2016/17 clinical audit, focusing on the performance of Emergency Departments (EDs) against audit standards. The audit objectives include benchmarking current performance, allowing national and peer comparisons, identifying areas for improvement,
2 views • 45 slides
Understanding Quality Assurance in Public Audit Systems
Quality assurance in public audit systems plays a crucial role in evaluating audit compliance, improving quality control processes, and detecting problem areas. It helps ensure effective operation and establishment of quality controls, supporting audit findings with proper evidence, disseminating go
1 views • 22 slides
Understanding Medication Errors in Pharmacovigilance Centers
Adverse drug reactions resulting from medication errors are a significant concern faced by pharmacovigilance centers. This content discusses the importance of this problem, contributing factors, the relevance to pharmacovigilance centers, and strategies for managing medication errors. It highlights
5 views • 29 slides
Internal Audit Planning and Practices for Effective Risk Management
Planning an internal audit following EC practices is crucial for enhancing and protecting organizational value. The Internal Audit Service's mission focuses on providing risk-based assurance and advice to improve risk management, control, and governance processes. From audit engagement to kick-off,
0 views • 17 slides
Weatherization Energy Auditor Single Family - Energy Audit Software
This content discusses the standard curriculum for the Weatherization Assistance Program focusing on energy audit software for single-family homes. It covers learning objectives, terminology, energy audits, federal rules, and guidance related to energy audit approval procedures. Participants will le
0 views • 14 slides
Audit Practices for SDGs Implementation Evaluation in Turkey
This content delves into the process of auditing the implementation of Sustainable Development Goals (SDGs) in Turkey, focusing on renewable energy policies. It covers topics such as audit perspective, selecting audit topics, the link between national targets and SDGs, planning stages, defining audi
1 views • 22 slides
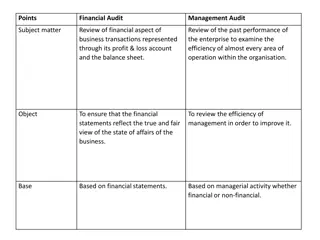
Comparison of Financial Audit and Management Audit
Financial audit focuses on reviewing financial statements for accuracy and compliance, while management audit assesses the efficiency of operations and future planning. Financial audit is a legal requirement for registered companies, conducted by chartered accountants, and emphasizes error detection
0 views • 17 slides
Understanding Departmental Audits in GST
Departmental audits in GST involve the examination of records, returns, and other documents to verify the correctness of turnover declared, taxes paid, refunds claimed, and input tax credit availed. This audit ensures compliance with the provisions of the CGST Act, 2017. Types of audits under GST in
7 views • 27 slides
Clinical Audit Results of Moderate to Acute Severe Asthma in EDs 2016/17
This clinical audit presentation showcases the national results of how Emergency Departments (EDs) are performing against the established audit standards for moderate to acute severe asthma in the year 2016/17. The objectives of the audit were to benchmark current performance, enable national and pe
1 views • 36 slides
Understanding Internal Audit and Controls Process
This content provides an overview of the pre-audit presentation, objectives of the presentation, the definition of internal audit, the role of internal audit in examining university departments, the university audit process, internal audit reporting lines, and insights on internal controls in an org
3 views • 30 slides
Understanding Adverse Information Reporting in National Security
This detailed content covers the importance of reporting adverse information regarding cleared employees in national security settings. It outlines the impact of adverse information on security clearances, the 13 adjudicative guidelines used in determining eligibility for sensitive duties, NISPOM re
0 views • 15 slides
Internal Audit Department Overview
The Internal Audit Department at the University of North Carolina Charlotte is led by Chief Audit Officer Jennifer Walker and Internal Audit Manager Kevin Vehar. The team provides risk-based assurance, advisory services, and investigations to enhance organizational value. Their mission is to offer o
1 views • 17 slides
Conducting an Effective School Energy Audit
Performing an energy audit at a school helps in understanding energy usage patterns, identifying areas of waste, and creating energy-saving action plans. The audit involves collecting data, creating switch-off lists, and filling out templates methodically to track energy consumption. It should be do
0 views • 16 slides
Reforming Audit Requirements for Federal Awards
This presentation outlines the major policy changes in the government-wide requirements for auditing Federal awards under the Single Audit Act Amendments of 1996. It discusses the transition from OMB Circular A-133 to Subpart F-Audit Requirements in 2 CFR Part 200, focusing on targeting audit requir
1 views • 34 slides
Federal Audit Executive Council - Contract Closeout Guide Highlights
The Federal Audit Executive Council (FAEC) plays a vital role in coordinating issues affecting the Federal audit community, with a key emphasis on audit policy and operations. The FAEC's contracting committee has developed audit guides covering various topics like acquisition planning, market resear
1 views • 12 slides
Essential Tips for a Successful Audit Process
Learn key insights for effectively preparing and participating in an audit process, including understanding annual audit requirements, the importance of the initial steps, maximizing the audit process, and crucial steps for a successful audit. Gain a comprehensive overview to streamline your organiz
0 views • 17 slides
Analysis of Atopic Eczema in Children: A National Clinical Audit Program
The British Association of Dermatologists conducted a national clinical audit program in 2015 to evaluate atopic eczema in children based on NICE guidelines. The audit included contributions from BAD and BSPD members, as well as private providers. It involved 128 submissions from 98 centers, with 12
2 views • 18 slides
Key Considerations for a Successful Audit Process in Nairobi
Understanding the importance of audit file preparation and key considerations for a successful audit process in Nairobi. Key staff participation, management of audit process, sharing draft financial statements for quality review, and following Act requirements are crucial steps for a successful audi
0 views • 23 slides
Stress Ulcer Prophylaxis in ICU - Adverse Reactions Reporting Guidelines
Guidelines for reporting adverse reactions in Stress Ulcer Prophylaxis in the Intensive Care Unit (SUP-ICU), including definitions of Adverse Reactions (AR), Serious Adverse Reactions (SAR), Adverse Events (AE), Serious Adverse Events (SAE), SARs in SUP-ICU, and SUSARs. Specific conditions considere
0 views • 8 slides
Understanding Audit Risk Assessment Process
Learn about the audit risk assessment process, including objectives, risk assessment techniques, and the PPC audit approach. Understand preliminary engagement activities, client acceptance/continuance considerations, and important documentation. Explore how to assess risks of material misstatement a
0 views • 61 slides
Insights on Constructing Geopolitical Risk Audit
Details on constructing the audit for the paper "Measuring Geopolitical Risk" by Dario Caldara and Matteo Iacoviello are outlined. It covers the process of building the GPR index, designing the audit sample, and coding articles to identify geopolitical risks discussed in newspapers. The methodology
0 views • 17 slides
Auditing Lowballing: Impact on Audit Quality and Recovery
The study explores the practice of lowballing audit fees and its effects on auditor independence and audit quality. Key research questions examine the relationship between non-audit fees, client importance, and lowballing, as well as the recovery of initial fee discounts. Hypotheses suggest connecti
0 views • 12 slides
Understanding Single Audit Requirements for Hawaii Child Nutrition Programs 2016
In accordance with federal regulations, non-Federal entities that expend $750,000 or more in Federal funds, including USDA's child nutrition programs, are required to undergo a Single Audit. The audit must be completed within nine months of the organization's fiscal year-end, and the final report mu
0 views • 5 slides
Understanding Adverse Events and Unanticipated Problems in Clinical Research
Explore the significance of adverse event reporting in clinical research, including definitions of adverse events (AEs) and serious adverse events (SAEs), as well as unanticipated problems (UAPs). Learn about the responsibilities of Investigators, Clinical Research Coordinators (CRCs), and Sponsors
0 views • 63 slides
Continuous Audit at Insurance Companies
Creating a rule-based model for anomaly detection, this research focuses on developing an architecture for continuous audit systems. By utilizing historical data, the scope includes detecting fraud, discrepancies, and internal control weaknesses, leading to a maturity model for automated continuous
0 views • 27 slides
Overview of UN-Habitat Audit Progress and Best Practices 2020-2021
The UN-Habitat audit for 2020-2021 showcases adherence to international best practices, including distinct internal/external audit functions, risk-based methodology, and operational independence. Audit coverage spans regional, country, and global operations, with critical business processes under sc
0 views • 9 slides
Brain Oxygen Optimization in Severe TBI: Adverse Events Analysis
This study led by Dr. Robert Silbergleit focuses on adverse events in brain oxygen optimization for severe Traumatic Brain Injury (TBI) patients. It outlines key points for reporting adverse events, discusses relatedness algorithms, and presents scenarios to analyze adverse events occurrence post-en
0 views • 24 slides