Understanding Proteins: Functions, Structure, and Breakdown
Explore the world of proteins, from their essential functions in living organisms to the chemistry of amino acids that form them. Learn how proteins are structured, the significance of amide links, and how they are broken down through hydrolysis. Delve into the importance of essential amino acids and how our diet plays a crucial role in providing them.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
a) Function of proteins Learning intention Learn about the function of proteins in living things.
b) Amino Acids Learning intention Learn chemistry of amino acids, the building blocks of proteins. about the characteristic
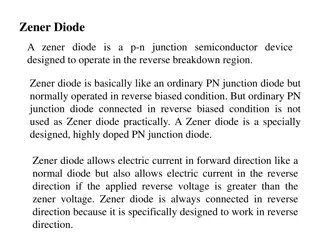
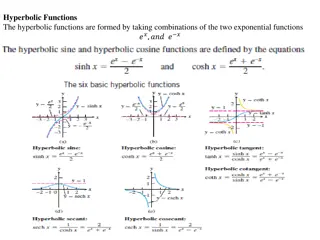
Amino acids All proteins contain the elements C,O,H, N. They are condensation polymers, made by amino acids linking together. The body cannot make every type of amino acids that it needs. So our diet must contain essential amino acids. (about 10 of them). We synthesis the others. Amino Acids R Most proteins contain 20+ different amino acids R H O NH2CHCOOH H N C C O H H When R is Hydrogen, the amino acid is glycine (Gly) (aminoethanoic acid) When R is CH3, the amino acid is alanine (Ala) (2-aminopropanoic acid)
c) Amide links Learning intention Learn how proteins are formed by condensation reactions of amino acids to produce amide (peptide) links.
Protein Polymers Proteins are condensation polymers, made by amino acids linking together. An amine group of one molecule links to the carboxyl group of another molecule to form an amide link or peptide bond.
Protein Polymers CH3 H CH3 H H H O OH O OH + O OH + N C - C N C - C N C - C H H H H H H glycine alanine alanine Tripeptide, ala-gly-ala H H H CH3 CH3 O O H O OH N C C - N C - C N C C - + 2H2O H H H H Polypeptide chain can have 10000 amino acids amide (peptide) link
d) Hydrolysis of protein Learning intention Learn how proteins are broken down to amino acids by the process of hydrolysis.
Essential Amino acids The body cannot make all the amino acids required for body proteins and is dependent on dietary protein for supply of certain amino acids known as essential amino acids
Protein hydrolysis Proteins are broken down during digestion. Digestion involves the hydrolysis of proteins to form amino acids Protein + 2H2O Amino acids
Experiment 1. Place the test tube in a water bath and leave until the contents reach the same temperature as the water bath. 2. Use the 2 cm3 syringe to measure out 1 cm3 of lipase from the beaker in the water bath for the temperature you are investigating. 3. Add the lipase to the test tube and start the stopclock/ stopwatch. 4. Stir the contents of the test tube until the solution loses its pink colour.
Explanation Phenolphthalein is an indicator that is pink in alkaline solutions of about pH10. When the pH drops below pH 8.3 phenolphthalein goes colourless. Here, an alkaline solution of milk, lipase and phenolphthalein will change from pink to colourless as the fat in milk is broken down to form fatty acids (and glycerol) thus reducing the pH to below 8.3. The time taken for this reaction to occur is affected by temperature.
Changes in protein structure on heating Within proteins, the long chain molecules may be twisted to form spirals, folded into sheets, or wound around to form other complex shapes. The chains are held in these forms by intermolecular bonding between the side chains of the constituent amino acids. When proteins are heated, during cooking, these intermolecular bonds are broken allowing the proteins to change shape (denature).These changes alter the texture of foods.
Enzyme Activity Enzymes catalyse chemical reactions in the body. Each enzyme has a unique shape held together by many weak bonds. Changes to pH and temperature can denature the enzyme. This changes the enzymes shape stops it working properly. Narrow optimum range Enzyme activity Temp or pH The bonds that hold most biological enzymes are broken around 60oC.