Understanding Electromagnetic Radiation and Quantum Energy in Chemistry
Delve into the fundamentals of atomic structure, periodicity, and electromagnetic radiation in chemistry. Explore the concepts of wavelength, frequency, speed of light, and quantum energy. Learn about important terms, example problems, Max Planck's contributions, and the photoelectric effect. Unravel the interplay between energy, frequency, and wavelength in the realm of chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Atomic Structure and Periodicity DE Chemistry Dr. Walker
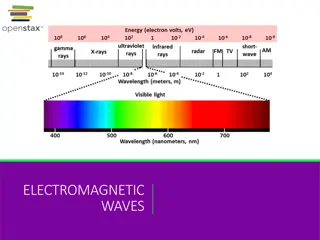
Electromagnetic Radiation Energy traveling in waves Three properties Wavelength, Frequency, and Speed
Important Terms File:Wavelength.png Wavelength ( ) Distance between consecutive peaks or troughs in a wave
Important Terms File:Wavelength.png Frequency ( ) Number of waves that pass through a given point in a second Measured in hertz (Hz = s-1)
Important Terms File:Wavelength.png Speed Electromagnetic radiation travels at the speed of light = 3.0 x 108m/s wavelength x frequency = speed of light ( = c)
Example Problem What is the wavelength for a radio station with a frequency of 98.7 MHz?
Example Problem What is the wavelength for a radio station with a frequency of 98.7 MHz? Remember = c 98.7 MHz = 9.87 x 107Hz (or s-1) = If = c , then = c/ = (3.0 x 108m/s)/(9.87x 107Hz) = 3.0 m
Max Planck Showed that energy could only be emitted in multiples of the frequency , called a quantum Planck s constant, h = 6.626 x 10-34J s Energy, E = h
Example What is the energy of the wavelength emitted by the radio station broadcasting at 98.7 MHz?
Example What is the energy of the wavelength emitted by the radio station broadcasting at 98.7 MHz? E = h E = (6.626 x 10-34J S) (9.87 x 107s-1) E = 6.54 x 10-26J
Photoelectric Effect The photoelectric effect refers to the phenomenon in which electrons are emitted from the surface of a metal when light strikes it. Using relationships between kinetic energy of electrons and the wavelength-frequency relationship, he developed the theory or relativity (E = mc2) in 1905 and received a Nobel prize. This suggested that light acts like a particle and has mass.
Louis de Broglie Light travels through space as a wave Light transmits energy as a particle Particles have wavelength, exhibited by diffraction patterns = wavelength, v= velocity (this isn t !) h= Planck s constant, m = mass Large particles have very short wavelengths All matter exhibits both particle and wave properties
The Spectrum When light is passed through a prism, a rainbow is produced which contains all wavelength of visible light
Atomic Hydrogen Spectrum When a sample of hydrogen gas receives a high-energy spark, the H2 molecules absorb energy, and some of the H-H bonds are broken. The resulting hydrogen atoms are excited; meaning they contain excess energy This energy is released by emitting light of various wavelengths to produce what is called the emission spectrum of the hydrogen atom.
Emission Spectrum Of Hydrogen Remember wavelength can be related to energy (E = hc/ ) Shows energy release only occurs at specific levels (quantum of energy)
Niels Bohr (18851962) Based on Planck s work and hydrogen emission spectra, he proposed that the electron in a hydrogen atom moves around the nucleus only in certain allowed circular orbits. This was consistent with Planck s quantum of energy Could calculate energy changes from exciting atoms and allowing them to return to theground state(lowest possible energy)
Bohrs Model Problem: The calculations only worked with hydrogen Historically important, but incorrect overall
The Quantum Model De Broglie and Schrodinger: The Electron as a standing wave Standing waves do not propagate through space Standing waves are fixed at both ends Only certain size orbits can contain whole numbers of half wavelengths, otherwise interference occurs Fits the observation of fixed energy quantities - "quanta"
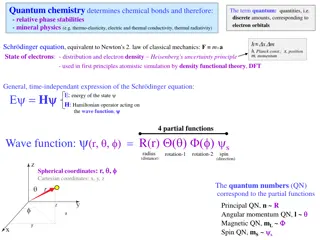
Schrodinger Equation This is actually a simplified equation Operators concern the energy (E) for the electron when applied to the wave function Wave functions concern the electron s three- dimensional position in space (x,y,z on a graph) Orbitals Orbitals are not circular orbits for electron Orbitals are areas of probability for locating electrons
Heisenbergs Uncertainty Principle "There is a fundamental limitation on how precisely we can know both the position and momentum of a particle at a given time The more accurately we know the position of any particle, the less accurately we can know its momentum, and vice-versa
Physical Meaning Of A Wave Function Square of the absolute value of the wave function gives a probability distribution. The quotient N1/N2 is the ratio of the probabilities of finding the electron at positions 1 and 2 For example, if the value of the ratio N1N2 is 100, the electron is 100 times more likely to be found at position 1 than at position 2.
Probability Distributions Think about this diagram is as a three dimensional time exposure with the electron as a tiny moving light. When an electron hits point, it s an exposure More hits, darker exposure The darker the point, the higher the probability of an electron being there at any particular time Finding probability distributions using wave functions are how orbital shapes are determined. Probability Distribution for a 1s orbital
Quantum Numbers We ve learned that orbitals are areas within the atom where electrons are likely (have a high probability) of being located at a given point in time Each orbital (or wave function) represents a solution for the Schrodinger equation This involves a set of four quantum numbers Each electron has a unique set of quantum numbers
Quantum Numbers The principal quantum number (n) has integral values: 1, 2, 3, . . Based on the size and energy of the orbital Corresponds to the rows on the periodic table Number of electrons that can fit in an energy level = 2n2
Quantum Numbers The angular momentum quantum number ( ) has integral values from 0 to n 1 for each value of n. For example, For n=2, can be equal to 0 and 1 (=n 2). For n=3, can be equal to 0, 1, and 2 This represents the possible orbital types = 0 is a s orbital = 1 is a p orbital = 2 is a d orbital = 3 is a f orbital
Quantum Numbers The magnetic quantum number (m ) has integral values between and - , including zero. This represents the specific orbital within the energy level For example, let s say n=2 and =1. We have a 2p orbital. The 2p energy level holds 6 electrons, with three specific orbitals. The three possible m values are -1, 0, and 1. Each number represents one of the three orbitals in that energy level.
Quantum Numbers The electron spin quantum number (ms), can have only one of two values, +1/2 or -1/2 We can interpret this to mean that the electron can spin in one of two opposite directions, with or against a magnetic field. This relates to the two electrons that can fit into one specific orbital in a given energy level
Pauli Exclusion Principle Old Definition Two electrons in same orbital have different spins (which is true, just incomplete) New Definition Each electron has a unique set of four quantum numbers
Quantum Numbers If you haven t guessed by now This is the true quantum mechanical basis for electron configurations
Orbital Shapes and Energies Size of orbitals as the surface that contains 90% of the total electron probability Orbitals of the same shape (s, for instance) grow larger as n (principal quantum number, energy level) increases
Orbital Shapes and Energies s Orbitals Spherical shape Nodes (s orbitals of n=2 or greater) Internal regions of zero probability
Orbital Shapes and Energies p Orbitals Two lobes each Occur in levels n=2 and greater Each orbital lies along an axis (2px, 2py, 2pz)
Orbital Shapes and Energies D orbitals Occur in levels n=3 and greater Two fundamental shapes Four orbitals with four lobes each, centered in the plane indicated in the orbital label dxz dyz dxy dx2- y2 Fifth orbital is uniquely shaped - two lobes along the z axis and a belt centered in the xy plane, dz2
Orbital Shapes and Energies F orbitals Occur in levels n=4 and greater Highly complex shapes Not involved in bonding in most compounds
Orbital Energies All orbitals with the same value of n have the same energy "degenerate orbitals" (hydrogen only!) The lowest energy state is called the ground state When the atom absorbs energy, electrons may move to higher energy orbitals - excited state
Notice the degenerate orbitals on the left. The orbitals are NOT degenerate on the right.
Orbital Energies Atoms other than hydrogen have variations in energy for orbitals having the same principal quantum number Based on repulsions on electrons with each other in elements with > 1 electron (everyone but hydrogen) Electrons fill orbitals of the same n value in preferential order Ens < Enp < End < Enf Electron density profiles show that s electrons penetrate to the nucleus more than other orbital types Closer proximity to the nucleus = lower energy
Periodic Table History John Newlands Law of octaves Idea that elements repeat properties every 8th element Didn t work because it didn t account for transition metals Dmitri Mendeleev Arranged by atomic number Successfully predicted existence of undiscovered elements, such as germanium, gallium, and scandium
Glenn Seaborg Synthesized several actinides (aka transuranium elements) using particle accelerators Made suggestion that transuranium elements should be removed from the transition metals Placed underneath periodic table in their present position
Electron Configuration Aufbau Principle "As protons are added one by one to the nucleus to build up elements, electrons are similarly added to these hydrogen-like orbitals Hund's Rule "The lowest energy configuration for an atom is the one having the maximum number of unpaired electrons allowed by the Pauli principle in a particular set of degenerate (same energy) orbitals
Electron Configurations We can more thoroughly review these if necessary
Shorthand Shorter form of electron configuration [Ne] = 1s22s22p6 [Ar] = 1s22s22p63s23p6 Potassium Atomic Number = 19 1s22s22p63s23p64s1 [Ar]4s1
Exceptions Unusual trends have been discovered in the transition metals Half-filled orbitals give higher stability Completely filled orbitals give higher stability Examples Cr expected [Ar]4s23d4, actual [Ar]4s13d5 Cu expected [Ar]4s23d9, actual [Ar]4s13d10
Periodic Table Vocabulary Valence Electrons Electrons in the outermost principal quantum level of an atom Elements in the same group (vertical column) have the same valence electron configuration Transition metals What we have called the "d" block, groups 3-12 Lanthanide and Actinide Series The sets of 14 elements following lanthanum and actinium What we have called the "f" block
Periodic Table Vocabulary Main-group or Representative Elements Groups 1-2, 13-18 Configurations are consistent Metalloids (semi-metals) Found along the border between metals and nonmetals Exhibit properties of metals and nonmetals