Understanding Chemical Quantities: The Mole Concept and Molar Mass
Chemists use the mole concept to relate mass and the number of atoms in chemical reactions. Avogadro's number, molar mass, stoichiometry, and energy changes in reactions are key concepts explored in this chapter. The mole is a vital unit in chemistry, enabling scientists to quantify substances and make accurate calculations in various chemical processes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Chapter 6B Chemical Quantities 1
CHAPTER OUTLINE The Mole Concept Molar Mass Calculations Using the Mole Stoichiometry & Molar Ratios Mole-Mole & Mass-Mole Calculations Mass-Mass Calculations Limiting Reactant Percent Yield Energy Changes in Chemical Reactions 2
THE MOLE CONCEPT Chemists find it more convenient to use mass relationships in the laboratory, while chemical reactions depend on the number of atoms present. In order to relate the mass and number of atoms, chemists use the SI unit mole (abbreviated mol). 3
THE MOLE CONCEPT The number of particles in a mole is called Avogadro s number and is 6.02x1023. 6.02 x 1023 equals to 1 mole Avogadro s number (NA) 4
THE MOLE CONCEPT A mole is a very large quantity 6.02x1023 If 10,000 people started to count Avogadro s number and counted at the rate of 100 numbers per minute each minute of the day, it would take over 1 trillion years to count the total number. 5
THE MOLE CONCEPT 1 mole H atoms = 6.02x1023 H atoms 1 mole H2 molecules = 6.02x1023 H2 molecules = 2 x (6.02x1023) H atoms 1 mole H2O molecules = 6.02x1023 H2O molecules = 2 x (6.02x1023) H atoms = 6.02x1023 O atoms 1 mole Na+ ions = 6.02x1023 Na+ ions 6
THE MOLE CONCEPT The atomic mass of one atom expressed in amu is numerically the same as the mass of 1 mole of atoms of the element expressed in grams. Mass of 1 H atom = 1.008 amu Mass of 1 Mg atom = 24.31 amu Mass of 1 Cl atom = 35.45 amu Mass of 1 mol H atoms = 1.008 grams Mass of 1 mol Mg atoms = 24.31 g Mass of 1 mol Cl atoms = 35.45 g 7
MOLAR MASS The mass of one mole of a substance is called molar mass, and is measured in grams. Mass of one mole of H2O 2 mol H atoms = 2 (1.01 g) = 2.02 g 1 mol O atom = 1 (16.00 g) = 16.00 g Molar mass 18.02 g 8
MOLAR MASS Mass of one mole of Ca(OH)2 1 mol Ca atom = 1 (40.08 g) = 40.08 g 2 mol O atoms = 2 mol H atoms = 2 (16.00 g) = 32.00 g 2 (1.01 g) = 2.02 g Molar mass 74.10 g 9
Example 1: Calculate the molar mass of each compound shown below: Lithium carbonate Li2CO3 Li C O 2 x 6.94 = 13.88 g 1 x 12.01 = 12.01 g 3 x 16.00 = 48.00 g Molar mass = 73.89 g/mol 10
Example 2: Calculate the molar mass of each compound shown below: Salicylic acid C7 H6O3 C H O 7 x 12.01 = 84.07 g 6 x 1.01 3 x 16.00 = 48.00 g = 6.06 g Molar mass = 138.13 g/mol 11
CALCULATIONS USING THE MOLE Conversions between mass, mole and particles can be done using molar mass and Avogadro s number. Avogadro s number Molar mass Mass of a substance Moles of a substance Particles of a substance MM NA 12
Example 1: How many moles of iron are present in 25.0 g of iron? mole g 55.85 1 25.0 g Fe x mol Fe 0.448 = Molar mass 3 significant figures 13
Example 2: What is the mass of 5.00 mol of water? g mol= 1 18.02 5.00 mol H O x g H O 90.1 2 2 3 significant figures Molar mass 14
Example 3: How many Mg atoms are present in 5.00 g of Mg? mass mol atoms atoms mol 1 mol g 24.30 1 6.02 x 1023 5.00 g Mg x x = 1.24x1023 atoms Mg Avogadro s number Molar mass 3 significant figures 15
Example 4: How many molecules of HCl are present in 25.0 g of HCl? mass mol molecules molecules mol 1 mol g 36.46 1 6.02 x 1023 25.0 g HCl x x = 4.13 x 1023 molecules HCl 3 significant figures 16
MOLES OF ELEMENTS IN A FORMULA The subscripts in a chemical formula of a compound indicate the number of atoms of each type of element. For example, 1 molecule of aspirin contains: C9H8O4 8 H atoms 9 C atoms 4 O atoms 17
MOLES OF ELEMENTS IN A FORMULA The subscripts also indicate the number of moles of each element in one mole of the compound. For example, 1 mole of aspirin contains: C9H8O4 9 mole C atoms 8 mole H atoms 4 mole O atoms 18
MOLES OF ELEMENTS IN A FORMULA Using the subscripts from the aspirin formula, one can write the following conversion factors for each of the elements in 1 mole of aspirin. C9H8O4 9 moles C 1 mole C9H8O4 8 moles H 1 mole C9H8O4 4 moles O 1 mole C9H8O4 19
Example 1: Determine the number of moles of C atoms in 1 mole of each of the following substances: a) Acetoaminophen used in Tylenol C8 H9NO2 8 moles C 1 mole C8H9 NO2 b) Zinc dietary supplement Zn(C2 H3O2)2 4 moles C 1 mole Zn(C2 H3O2)2 20
Example 2: How many carbon atoms are present in 1.50 moles of aspirin, C9H8O4? 9 moles C 1 mole C9H8 O4 x6.02x1023 atoms 1 mole C 1.50 mol C9H8O4x = 8.13x1024 atoms 21
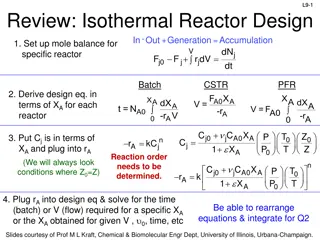
SUMMARY OF MASS-MOLE CALCULATIONS 22
STOICHIOMETRY Stoichiometry is the quantitative relationship between the reactants and products in a balanced chemical equation. A balanced chemical equation provides several important information about the reactants and products in a chemical reaction. 23
MOLAR RATIOS For example: 1 N2 (g) + 3 H2 (g) 2 NH3 (g) 3 molecules This is the molar ratios between the reactants and products 1 molecule 2 molecules 100 molecules 300 molecules 200 molecules 106 molecules 3x106 molecules 2x106 molecules 1 mole 3 moles 2 moles 24
Example 1: Determine each mole ratio below based on the reaction shown: 8 CO2 + 10 H2O 2 C4H10 + 13 O2 mol O mol CO 13 8 = 2 2 mol C H mol H O 2 10 = 4 10 2 25
STOICHIOMETRIC CALCULATIONS Stoichiometric calculations can be classified as one of the following: calculations Mass-mass Mass-mole calculations MASS of compound B MASS of compound A Mole-mole calculations MM MM MOLES of compound A MOLES of compound B molar ratio 26
MOLE-MOLE CALCULATIONS Relates moles of reactants and products in a balanced chemical equation MOLES of compound A MOLES of compound B molar ratio 27
Example 1: How many moles of nitrogen will react with 2.4 moles of hydrogen to produce ammonia as shown in the reaction below? 1 N2 (g) + 3 H2 (g) 2 NH3 (g) 1 mol N x mol H 3 2 2.4 mol H2 = 0.80 mol N2 2 Mole ratio 28
Example 2: How many moles of ammonia can be produced from 32 moles of hydrogen? (Assume excess N2 present) 1 N2 (g) + 3 H2 (g) 2 NH3 (g) mol NH mol H 3 2 x 3 32 mol H2 = 21 mol NH3 2 Mole ratio 29
Example 3: In one experiment, 6.80 mol of ammonia are prepared. How many moles of hydrogen were used up in this experiment? 1 N2 (g) + 3 H2 (g) 2 NH3 (g) mol H x mol NH 2 3 2 6.80 mol NH3 = 10.2 mol H2 3 Mole ratio 30
MASS-MOLE CALCULATIONS Relates moles and mass of reactants or products in a balanced chemical equation MASS of compound A MM MOLES of compound A MOLES of compound B molar ratio 31
Example 1: How many grams of ammonia can be produced from the reaction of 1.8 moles of nitrogen with excess hydrogen as shown below? 1 N2 (g) + 3 H2 (g) 2 NH3 (g) mol NH mol N 1 g NH x mol NH 1 17.04 2 1.8 mol N2 x 3 3 = 61 g NH3 2 3 Mole ratio Molar mass 32
Example 2: How many moles of hydrogen gas are required to produce 75.0 g of ammonia? 1 N2 (g) + 3 H2 (g) 2 NH3 (g) 1 3 mol H x mol NH 2 mol NH g NH 17.04 75.0 g NH3 x 2 3 = 6.60 mol H2 3 3 Molar mass Mole ratio 33
Example 3: How many moles of ammonia can be produced from the reaction of 125 g of nitrogen? 1 N2 (g) + 3 H2 (g) 2 NH3 (g) 1 2 mol NH mol N 1 mol N g N 28.02 125 g N2 x x 3 2 = 8.92 mol NH3 2 2 Molar mass Mole ratio 34
MASS -MASS CALCULATIONS Relates mass of reactants and products in a balanced chemical equation 35
Example 1: What mass of oxygen will be required to react completely with 96.1 g of propane, C3H8, according to the equation below? 1 C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (g) Moles of oxygen Mass of propane Moles of propane Mass of oxygen 36
Example 1: 1 C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (g) 1 5 mol C H x g C H 44.11 mol O x mol C H 1 g O x mol O 1 3 8 2 96.1 g C3H8 3 8 3 8 32.00 2 = 349 g O2 2 Molar mass Mole ratio Molar mass 37
Example 2: What mass of carbon dioxide will be produced from the reaction of 175 g of propane, as shown? 1 C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (g) Moles of carbon dioxide Mass of propane Moles of propane Mass of carbon dioxide 38
Example 2: 1 C3H8 (g) + 5 O2 (g) 3 CO2 (g) + 4 H2O (g) 1 3 mol C H x g C H 44.11 mol CO x mol C H 1 3 8 2 175 g C3H8 3 8 3 8 g CO x mol CO 1 44.01 2 = 524 g CO2 2 Molar mass Molar mass Mole ratio 39
LIMITING REACTANT When 2 or more reactants are combined in non- stoichiometric ratios, the amount of product produced is limited by the reactant that is not in excess. This reactant is referred to as limiting reactant. When doing stoichiometric problems of this type, the limiting reactant must be determined first before proceeding with the calculations. 40
LIMITING REACTANT ANALOGY Consider the following recipe for a sundae: 41
LIMITING REACTANT ANALOGY How many sundaes can be prepared from the following ingredients: amount of syrup, the limiting reactant. The number of sundaes possible is limited by the Limiting reactant Excess reactants 42
GUIDE TO LIMITING REACTANT CALC. Assume reactant 1 is limiting Assume reactant 2 is limiting Compare 43
Example 1: How many moles of H2O can be produced by reacting 4.0 mol of hydrogen and 3.0 mol of oxygen gases as shown below: 2 H2 (g) + 1 O2 (g) 2 H2O (g) Mole-mole calculations Limiting reactant 44
Example 1: Correct answer 2 H2 (g) + 1 O2 (g) 2 H2O (g) Assume H2 is LR 2 mol H O mol H 2 4.0 mol H2 x 2 = 4.0 mol H2O 2 Correct assumption Assume O2 is LR 2 mol H O mol O 1 3.0 mol O2 = 6.0 mol H2O x 2 2 45
Example 2: A fuel mixture used in the early days of rocketry was a mixture of N2H4 and N2O4, as shown below. How many grams of N2 gas is produced when 100. g of N2H4 and 200. g of N2O4 are mixed? 2 N2H4 (l) + 1 N2O4 (l) 3 N2 (g) + 4 H2O (g) Limiting reactant Mass-mass calculations 46
Example 2: Assumes N2O4 is LR Assumes N2H4 is LR 47
Example 2: 2 N2H4 (l) + 1 N2O4 (l) 3 N2 (g) + 4 H2O (g) Assume N2H4 is LR 1 3 mol N H x g N H 32.06 mol N mol N H 2 x = 2 100. g N2H4 2 4 2 4 2 4 4.68 mol N2 48
Example 2: 2 N2H4 (l) + 1 N2O4 (l) 3 N2 (g) + 4 H2O (g) Assume N2O4 is LR 1 3 mol N mol N O 1 mol N O x g N O 92.02 x = 2 200. g N2O4 2 4 2 4 2 4 6.52 mol N2 49
Example 2: 2 N2H4 (l) + 1 N2O4 (l) 3 N2 (g) + 4 H2O (g) Assume N2H4 is LR 4.68 mol N2 Assume N2O4 is LR 6.52 mol N2 Correct amount N2H4 is LR 50