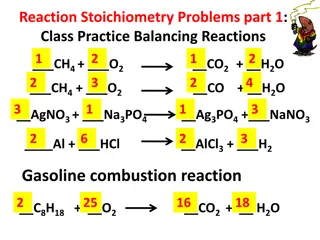
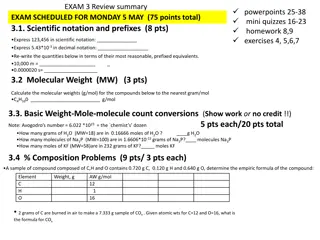
Understanding R.I.C.E. Tables and Stoichiometry for Limiting Reactants
R.I.C.E. tables play a crucial role in chemistry, particularly in stoichiometry when dealing with reactions and limiting reactants. This method involves organizing information and setting up equations to find unknowns. An example is provided with the combustion of ethene to determine the volume of carbon dioxide formed, demonstrating the step-by-step process using R.I.C.E. tables.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
USING R.I.C.E. TABLES AND STOICHIOMETRY WITH LIMITING REACTANTS
RICE tables are a common tool of chemists (college professors use then a lot!) to organize the information for stoichiometry with a reaction and set up mathematical equations when necessary to solve for unknowns. R = reaction (balanced) I = initial conditions (before the reaction) C = change (remove reactants, form products) E = end conditions (after the reaction)
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed?
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? BEWARE: Moles goes into a RICE table and moles will come out. To find volume will require a final step.
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? R =
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? R = C2H4 + 3O2 2CO2 + 2H2O
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? R = C2H4 + 3O2 2CO2 + 2H2O I =
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? R = C2H4 + 3O2 2CO2 + 2H2O I = 10. g
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? R = C2H4 + 3O2 2CO2 + 2H2O I = 10. g 0.625 mol ? 0 0
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? R = C2H4 + 3O2 2CO2 + 2H2O I = 10. g 0.625 mol ? 0 0 C =
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? R = C2H4 + 3O2 2CO2 + 2H2O I = 10. g 0.625 mol ? 0 0 C = -1x -3x +2x +2x
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? R = C2H4 + 3O2 2CO2 + 2H2O I = 10. g 0.625 mol ? 0 0 C = -1x all the ethene will be burned, so x = 0.63 mol -3x +2x +2x
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? R = C2H4 + 3O2 2CO2 + 2H2O I = 10. g 0.625 mol ? 0 0 C = -1x -0.625 mol -1.875 mol +1.25 mol +1.25 mol -3x +2x +2x
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? R = C2H4 + 3O2 2CO2 + 2H2O I = 10. g 0.625 mol ? 0 0 C = -1x -0.625 mol -1.875 mol +1.25 mol +1.25 mol -3x +2x +2x E =
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? R = C2H4 + 3O2 2CO2 + 2H2O I = 10. g 0.625 mol ? 0 0 C = -1x -0.625 mol -1.875 mol +1.25 mol +1.25 mol -3x +2x +2x E = 0 ?-1.875 mol 1.25 mol 1.25 mol
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? How many moles of CO2 was formed?
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? How many moles of CO2 was formed? 1.3 mol
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? How many moles of CO2 was formed? What volume of CO2 was formed? 1.3 mol
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? How many moles of CO2 was formed? What volume of CO2 was formed? 1.25 mol x 22.4 L/mol = 28 L 1.3 mol
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? How many moles of CO2 was formed? What volume of CO2 was formed? 1.25 mol x 22.4 L/mol = 28 L 1.3 mol What other information do we already know?
Example 1: 10. grams of ethene is combusted. What volume of carbon dioxide will be formed? How many moles of CO2 was formed? What volume of CO2 was formed? 1.25 mol x 22.4 L/mol = 28 L 1.3 mol What other information do we already know? Moles of O2 consumed Moles of H2O produced
Example 2: 10. grams of ethene is combusted with 50. grams of O2. What volume of carbon dioxide can be formed?
Example 2: 10. grams of ethene is combusted with 50. grams of O2. What volume of carbon dioxide can be formed? R = C2H4 + 3O2 2CO2 + 2H2O
Example 2: 10. grams of ethene is combusted with 50. grams of O2. What volume of carbon dioxide can be formed? R = C2H4 + 3O2 2CO2 + 2H2O I = 10. g 0.625 mol 1.56 mol 50. g
Example 2: 10. grams of ethene is combusted with 50. grams of O2. What volume of carbon dioxide can be formed? R = C2H4 + 3O2 2CO2 + 2H2O I = 10. g 0.625 mol 1.56 mol 50. g C = -1x WHICH X TO USE? SMALLEST X! -3x +2x +2x
Example 2: 10. grams of ethene is combusted with 50. grams of O2. What volume of carbon dioxide can be formed? R = C2H4 + 3O2 2CO2 + 2H2O I = 10. g 0.625 mol 1.56 mol 50. g C = -1x WHICH X TO USE? SMALLEST X! -3x +2x +2x If 0.625 = 1 x, then x = 0.625 If 1.56 = 3 x, then x = 0.521 SMALLEST
Example 2: 10. grams of ethene is combusted with 50. grams of O2. What volume of carbon dioxide can be formed? R = C2H4 + 3O2 2CO2 + 2H2O I = 10. g 0.625 mol 1.56 mol 50. g C = -1x -0.521 mol -1.56 mol +1.04 mol 1.04 mol -3x +2x +2x
Example 2: 10. grams of ethene is combusted with 50. grams of O2. What volume of carbon dioxide can be formed? R = C2H4 + 3O2 2CO2 + 2H2O I = 10. g 0.625 mol 1.56 mol 50. g C = -1x -0.521 mol -1.56 mol +1.04 mol 1.04 mol -3x +2x +2x E = 0.625 mol 0.521 mol 0.104 mol 0 1.56 mol 1.56 mol 1.04 mol 1.04 mol 1.04 mol 1.04 mol
Example 2: 10. grams of ethene is combusted with 50. grams of O2. What volume of carbon dioxide can be formed? What volume of CO2 is actually produced? 1.04 mols x 22.4 L/mol = 23 L
Example 2: 10. grams of ethene is combusted with 50. grams of O2. What volume of carbon dioxide can be formed? What volume of CO2 is actually produced? 1.04 mols x 22.4 L/mol = 23 L What else do we know? Moles of ethene left over Moles of water actually produced
Why use a RICE table for limiting reactant problems? How many RR tracks would it have taken?
Why use a RICE table for limiting reactant problems? How many RR tracks would it have taken? 2 to figure out which one is limiting
Why use a RICE table for limiting reactant problems? How many RR tracks would it have taken? 2 to figure out which one is limiting 1 more to find amount of excess
Why use a RICE table for limiting reactant problems? How many RR tracks would it have taken? 2 to figure out which one is limiting 1 more to find amount of excess 1 more for each of the additional products
Why use a RICE table for limiting reactant problems? How many RR tracks would it have taken? 2 to figure out which one is limiting 1 more to find amount of excess 1 more for each of the additional products It is your choice. We did 1 RICE table vs 4 RR Tracks. RICE tables will be necessary later. For now, it is optional.