Chemical equilibrium
Chemical equilibrium is a state where reactants and products reach a balance in a reaction. Learn about reversible and irreversible reactions, equilibrium constants, and how they affect chemical analysis. Discover the accuracy and applications of volumetric analysis, including titration methods like
3 views • 13 slides
Understanding Chemical Reactions: Reactants, Products, and Balancing Equations
Chemical reactions involve reactants that transform into products. Balancing equations is essential to ensure the conservation of mass. Learn about decomposition reactions, chemical equations, and methods to balance equations effectively.
4 views • 39 slides
Understanding Photosynthesis and Limiting Factors
Photosynthesis is an endothermic reaction that takes in energy from its surroundings. The law of limiting factors explains how various factors such as light intensity, temperature, and CO2 concentration can impact the rate of photosynthesis. Additionally, the concept of the inverse square law helps
7 views • 45 slides
Chemical Kinetics: Understanding Reaction Rates and Factors
Chemical kinetics is a branch of physical chemistry that explores the velocity and factors influencing chemical reactions. It studies how reactants transform into products, considering conditions like temperature, pressure, and reactant concentrations. Factors affecting reaction rates include the na
7 views • 24 slides
Understanding Chemical Reactions: Key Concepts and Practice Problems
Explore the fundamental concepts of chemical reactions, including how reactants and products are represented, the role of catalysts, and writing word equations. Learn through sample and practice problems illustrating different types of reactions. Master writing chemical equations for reactions invol
1 views • 51 slides
Understanding Stoichiometry in Chemistry
Stoichiometry in chemistry involves calculating quantities of reactants and products in a chemical reaction based on a balanced equation. This process ensures the conservation of mass and atoms. Mole ratios are used as conversion factors, and steps such as converting to moles and applying the mole r
1 views • 25 slides
Acetaminophen Synthesis Process Explained
Acetaminophen, also known as paracetamol, is a widely used analgesic and fever-reducing medicine. The synthesis of acetaminophen involves treating an amine with an acid anhydride to form an amide. This process includes mixing reactants, isolating crude acetaminophen, and purifying the final product.
0 views • 17 slides
Chemical Reactions: Vocabulary Practice and Experiments in Chemistry 101
In this additional text for practicing chemical reactions vocabulary, students in Chemistry 101 conduct an experiment by combining a solvent and a solute to observe a simple chemical reaction, focusing on the proper process and product description. The materials, instructions, and objectives are pro
0 views • 8 slides
Applications of Forces in Limiting Equilibrium for Solving Rigid Body Problems
Explore the principles of limiting equilibrium in rigid bodies with practical examples involving forces and friction. Learn how to analyze and solve problems, such as determining reaction forces and coefficients of friction, to ensure stability and balance in mechanical systems.
1 views • 8 slides
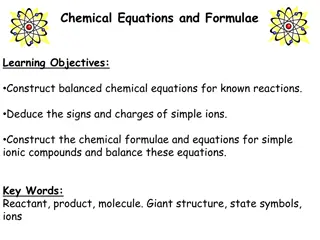
Understanding Chemical Equations and Formulae
Learn to construct balanced chemical equations for known reactions, deduce signs and charges of simple ions, and create chemical formulae for ionic compounds. Understand the concepts of reactants, products, molecules, giant structures, state symbols, and chemical formulas for various substances. Gai
3 views • 7 slides
Stoichiometry Test Review Sheet
A comprehensive review sheet covering key concepts in stoichiometry, including molar ratios, coefficients in chemical equations, determining actual yield in reactions, identifying limiting reactants, calculating percentage yield, and understanding mole ratios in chemical reactions. The review provid
0 views • 11 slides
Understanding Le Chatelier's Principle in Chemical Equilibrium
Le Chatelier's Principle states that when a system at equilibrium is disturbed by changes in concentration, temperature, or pressure, the equilibrium shifts to counteract the change. This principle can be applied to predict the direction of equilibrium when changes occur. Changes in concentration, p
0 views • 10 slides
Understanding Chemical Reactions and Reactivity Series
Chemical reactions involve the rearrangement of atoms, with reactants forming products. Different signs indicate a chemical reaction, such as gas release, odor, energy change, color change, and solid formation. Equations model these changes, showing the conservation of mass. Reactivity series help u
0 views • 6 slides
Understanding Chemical Reactions in Chemistry Lab
Explore the fascinating world of chemical reactions in the chemistry lab through hands-on experiments and theoretical knowledge. Learn to identify reactants and products, understand the characteristics of chemical reactions, use appropriate symbols in equations, and balance chemical equations effect
2 views • 22 slides
Understanding Different Types of Chemical Reactions
Chemical reactions involve the transformation of reactants into products. This comprehensive guide covers various types of reactions, including synthesis, decomposition, single-replacement, double-replacement, and combustion. Each reaction type is explained with examples and observable evidence such
0 views • 11 slides
Understanding Stoichiometry in Chemical Reactions
Stoichiometry is the concept of predicting the amounts of reactants and products in a chemical reaction, similar to following a recipe in cooking. It involves balancing chemical equations and determining the quantities of substances involved. By paying attention to coefficients, one can calculate ho
2 views • 65 slides
Understanding Limiting Reactants in Stoichiometry
In chemical reactions, the limiting reactant is crucial in determining the amount of product that can be formed. Learn how to identify the limiting reactant through mass-to-mass conversions using examples and practice problems. This process ensures optimal product yield based on the available reacta
1 views • 16 slides
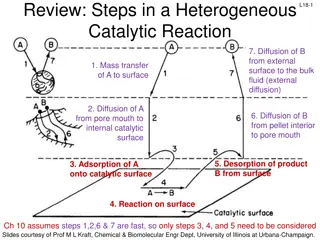
Understanding Heterogeneous Catalytic Reactions: Key Steps Explained
In a heterogeneous catalytic reaction, various important steps occur, including diffusion of reactants, adsorption onto the catalyst surface, surface reactions, and desorption of products. Different mechanisms like single-site, dual-site, and Eley-Rideal mechanisms are involved in the surface reacti
0 views • 17 slides
Calculating Limiting Reagent in Chemical Reactions
Calculating the amount of reactants in excess and the limiting reagent plays a crucial role in determining the maximum extent of a chemical reaction. By using the relative numbers of moles of substances as shown in balanced equations, one can identify the reactant that is fully utilized, hence limit
0 views • 14 slides
Understanding Photosynthesis: A Comprehensive Overview
Photosynthesis is the fundamental process through which plants produce food in the form of glucose. This intricate process involves the conversion of carbon dioxide and water into glucose using sunlight as the energy source. The key player in photosynthesis is chlorophyll, a green compound found in
0 views • 13 slides
Understanding Chemical Equilibrium in Reversible Reactions
Chemical equilibrium occurs when the concentrations of reactants and products remain constant over time in a reversible reaction. Reaction rate is proportional to concentration, and equilibrium is reached when the rate of formation equals the rate of consumption in both directions. Reversible reacti
0 views • 25 slides
Chemistry Exam Review: Topics in Scientific Notation, Molecular Weight, Stoichiometry, and Limiting Yield
Explore key concepts in chemistry, including scientific notation, molecular weight calculations, reaction balancing, stoichiometry, and limiting yield problems. Prepare for an upcoming exam by practicing various problems and conversions related to these topics, such as expressing numbers in scientif
0 views • 4 slides
Understanding Stoichiometry in Chemical Reactions
Stoichiometry in chemical reactions involves mass changes, limiting reactants, and calculating yields like moles and grams. Learn to solve problems involving different reactants and products, determining the quantities involved in a reaction. Examples cover decompositions, formations, and calculatio
0 views • 22 slides
Understanding Chemical Reactions and Catalysts
Chemical reactions involve the formation of new substances from reactants, with key processes like oxidation and reduction. Reversible reactions, endothermic and exothermic reactions, and the role of catalysts in speeding up reactions are explored. The significance of chemical symbols, formulas, and
0 views • 8 slides
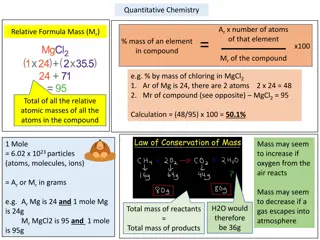
Understanding Quantitative Chemistry in Chemical Reactions
Explore the fundamentals of quantitative chemistry, from calculating percentages by mass to determining limiting reactants. Learn how to work out balanced symbol equations and solve concentration questions with ease. Enhance your knowledge of moles, molecular masses, and more in chemical reactions.
0 views • 4 slides
Understanding the Law of Mass Action in Chemical Reactions
Chemistry students often assume reactions go to completion, but the Law of Mass Action shows that equilibrium is reached with specific amounts of reactants and products. This law, demonstrated through experiments, helps determine equilibrium concentrations using the Keq expression. By applying basic
0 views • 18 slides
Understanding Limiting Factors in Ecosystems: A Case Study of Mono Lake
Explore the concept of limiting factors in populations through a study of Mono Lake's ecosystem. Discover how factors like light and temperature impact algae and brine shrimp populations, and analyze data from experiments conducted to understand population dynamics in controlled environments.
0 views • 9 slides
Interviewing Young Adults with Life-Limiting Conditions: Methodological Reflections
This study reflects on the methodological aspects of interviewing young adults with life-limiting conditions, emphasizing inclusive research practices. The research employs interpretive qualitative methods, focusing on unexpected consequences of pandemic control measures. Participants, including bot
0 views • 10 slides
Understanding Limiting Reactants in Chemistry
In chemistry, the limiting reactant is crucial as it determines the amount of product that can be formed in a reaction. By identifying and solving for the limiting reactant, you can find the maximum amount of product that can be obtained. This process involves understanding stoichiometry, calculatin
0 views • 16 slides
Understanding Oxidation-Reduction Reactions in Analytical Chemistry
Oxidation-reduction reactions play a crucial role in various chemical processes, including photosynthesis and corrosion. This content delves into the basics of redox reactions, explaining how electrons are transferred between reactants, leading to changes in oxidation numbers. Examples such as the r
0 views • 10 slides
Understanding Chemical Reactions, Formulas, and Equations
Explore the world of chemical reactions, formulas, and equations with insights into signs of reactions, chemical structure representation, and the balance of reactants and products in equations. Learn about how new substances are formed and the role of coefficients in balancing equations.
0 views • 5 slides
Understanding Energy in Chemical Reactions
Chemical reactions involve the release or absorption of energy in various forms like heat, light, sound, and electricity. Exergonic reactions release energy, while endergonic reactions absorb energy. Catalysts speed up reactions, while inhibitors slow them down without changing the amount of reactan
0 views • 8 slides
Understanding Chemical Reactions in Daily Life
Understanding chemistry, particularly chemical reactions, is crucial for our daily lives. Chemical reactions involve the transformation of substances into different ones, described by reactants and products in equations. By learning about chemical equations, word equations, formula equations, and th
0 views • 15 slides
Understanding R.I.C.E. Tables and Stoichiometry for Limiting Reactants
R.I.C.E. tables play a crucial role in chemistry, particularly in stoichiometry when dealing with reactions and limiting reactants. This method involves organizing information and setting up equations to find unknowns. An example is provided with the combustion of ethene to determine the volume of c
0 views • 35 slides
Understanding Homogenous Chemical Equilibrium
Homogenous chemical equilibrium occurs when reactants and products are in the same phase. This equilibrium remains independent of the volume of the reaction mixture. The concept is illustrated through the example of the Hydrogen-Iodide system and a generic reaction A + B --> 2C. Partial pressure pla
0 views • 56 slides
Radiation Protection in Diagnostic Radiography: Patient and Technologist Safety
Effective communication, immobilization, beam-limiting devices, beam filtration, gonadal shielding, exposure factors, IR combinations, good processing techniques, and reducing repeat radiographs are crucial for minimizing patient exposure during radiographic procedures. Equipment design features for
0 views • 49 slides
Explore Chemical Reactions with Sandwiches Activity
Engage in a hands-on activity relating sandwich-making to chemical reactions, focusing on concepts like limiting reactants and the Law of Conservation of Particles. Through visual representations and interactive questions, delve into the similarities between sandwich ingredients and chemical reactan
0 views • 25 slides
Understanding Chemical Reactions Through Sandwich Making
Explore the analogy of sandwich making to chemical reactions, learn about limiting reactants, and understand the Law of Conservation of Particles. Discover concepts through real-world examples and engage in interactive activities. Enhance your knowledge of chemistry in a fun and relatable manner.
0 views • 9 slides
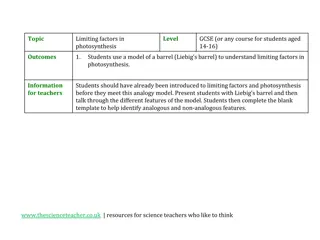
Understanding Limiting Factors in Photosynthesis Using Liebig's Barrel Model
Students aged 14-16 are introduced to limiting factors in photosynthesis through an analogy model of Liebig's barrel. The model illustrates how the rate of photosynthesis is limited by the factor that is least abundant, similar to filling a barrel with water. By identifying analogous and non-analogo
0 views • 4 slides
Supporting People Experiencing Homelessness with Life-Limiting Illnesses: A Research Perspective
This research discusses the support provided by staff in homeless accommodations for individuals facing homelessness and life-limiting illnesses. It covers the definitions and reasons for homelessness, the high risk of death in people experiencing homelessness, and common causes of mortality among t
0 views • 18 slides