Understanding Oxidation-Reduction Reactions in Chemistry
Explore the concept of oxidation and reduction in chemistry, which are fundamental processes that occur simultaneously in oxidation-reduction reactions. Learn about the role of oxygen, different types of oxidation reactions beyond burning, such as bleaching stains, and the concept of reduction involving the removal of oxygen from compounds. Discover how redox reactions involve electron transfer and are not limited to reactions involving oxygen.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
OXIDATION- REDUCTION REACTIONS REDOX
Oxygen Reaction i.e. oxidation Early chemists saw oxidation only as the combination of an element with oxygen to produce an oxide. burning of fuel is an oxidation reaction that uses oxygen. e.g. when methane (CH4), a component of natural gas, burns in air, it oxidizes and forms oxides of carbon and hydrogen. one oxide of carbon is carbon dioxide, CO2.
Not just Burning Not all oxidation involves burning. iron turns to rust, it oxidizes to compounds such as iron(III) oxide (Fe2O3).
Bleaching stains: Bleaching is oxidation liquid household bleach contains sodium hypochlorite (NaClO) releases oxygen oxidizes stains to a colorless form. Powdered bleaches may contain: calcium hypochlorite (Ca(ClO)2), sodium perborate (NaBO3), or sodium percarbonate (2Na2CO3 3H2O2).
Hydrogen peroxide (H2O2) also releases oxygen when it decomposes. It is both a bleach and a mild antiseptic that kills bacteria by oxidizing them. (insert dumb bar joke here)
Reduction opposite of oxidation. Originally, reduction meant the loss of oxygen from a compound. The reduction of iron ore to metallic iron involves the removal of oxygen from iron(III) oxide. The reduction is accomplished by heating the ore with carbon, usually in the form of coke.(what they make at Tonawanda Coke). 2Fe2O3+ 3C 4Fe + 3CO2
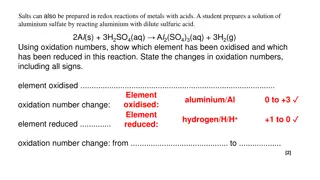
What is Redox? Oxidation and reduction always occur simultaneously. No oxidation occurs without reduction, and no reduction occurs without oxidation. The substance gaining oxygen is oxidized, while the substance losing oxygen is reduced. Reactions that involve these processes are therefore called oxidation-reduction reactions. are also known as redox reactions.
Electron Transfer Modern concepts of oxidation extend to include many reactions that do not involve oxygen. Oxygen is the most electronegative element next to fluorine It s an electron stealer As a result, when oxygen bonds with an atom of a different element (other than fluorine), electrons from that atom shift toward oxygen. Redox reactions are currently involve any shift of electrons between reactants.
New Definitions (must know) Oxidation - a process that involves complete or partial loss of electrons OR a gain of oxygen; it results in an increase in the oxidation number of an atom Reduction - a process that involves a complete or partial gain of electrons OR the loss of oxygen; it results in a decrease in the oxidation number of an atom
Memory aid (add to your ref tables): LEO says GER Lose Electrons Oxidation Gain Electrons Reduction
More confusing vocab oxidizing agent - the substance in a redox reaction that accepts electrons in the reaction, the oxidizing agent gets reduced Reduced = gains electrons reducing agent - the substance in a redox reaction that donates electrons in the reaction, the reducing agent gets oxidized Oxidized = loses electrons
Corrosion the reaction between a material, usually a metal, and its environment that produces a deterioration of the material and its properties. Iron, a common metal often used in the form of the alloy steel, corrodes by being oxidized to ions of iron by oxygen. Oxygen, the oxidizing agent, is reduced to oxide ions (in compounds such as Fe2O3) or to hydroxide ions. The following equations describe the corrosion of iron to iron hydroxides in moist conditions. 2Fe(s) + O2(g) + 2H2O(l) 2Fe(OH)2(s) 4Fe(OH)2(s) + O2(g) + 2H2O(l) 4Fe(OH)3(s)
Corrosion occurs more rapidly in the presence of salts and acids. Cars rust faster in where there is salt on roads These substances produce electrically conducting solutions that make electron transfer easier. The salt doesn t rust that car, it just makes it easier to rust faster. The corrosion of some metals can be a desirable feature. Verdigris
Corrosion Resistance Gold and platinum are noble metals because they are very resistant to losing their electrons by corrosion. Other metals lose electrons easily but are protected from extensive corrosion by the oxide coating formed on their surface. Aluminium oxidizes to form a coating of very tightly packed aluminium oxide particles. coating protects the aluminium object from further corrosion. Iron forms a coating when it corrodes, but the coating of iron oxide that forms is not tightly packed. Water and air can penetrate the coating and attack the iron metal below it. The corrosion continues until the iron object becomes only a pile of rust.
Controlling Corrosion corrosion of a steel support pillar of a bridge or the hull of an oil tanker is much more serious and costly. To prevent corrosion, the metal surface may be coated with oil, paint, plastic, or another metal. These coatings exclude air and water from the surface preventing corrosion. If the coating is scratched or worn away, the exposed metal will begin to corrode.
Another Control method one metal is sacrificed, allowed to corrode, in order to save another metal. e.g., to protect an iron object, a piece of magnesium (or another active metal ) may be placed in electrical contact with the iron. when oxygen and water attack the iron object, the iron atoms lose electrons as the iron begins to be oxidized. magnesium is a better reducing agent than iron (more easily oxidized - reference table J), the magnesium transfers electrons to the iron, preventing their oxidation to iron ions. So, the magnesium is sacrificed by oxidation and protects the iron in the process.
More example of sacrifice metals zinc and magnesium blocks can be attached to piers and ship hulls to prevent corrosion damage in areas submerged in water. Underground pipelines and storage tanks may be connected to magnesium blocks for protection. It is easier and cheaper to replace a block of magnesium or zinc than to replace a bridge or a pipeline.
Assigning Oxidation Numbers positive or negative number assigned to an atom to indicate its degree of oxidation or reduction. a bonded atom s oxidation number is the charge that it would have if the electrons in the bond were assigned to the atom of the more electronegative element.
Rules for Assigning Oxidation Numbers: refer to handout!
Rules For Oxidation Numbers 1 1. The oxidation number of a monatomic ion is equal in magnitude and sign to its ionic charge. Examples: the bromine ion is Br1-so the oxidation number is -1. The iron (III) ion is Fe3+so its oxidation number is +3. (Basically you are given the oxidation number with the charge on the ion.) Crack out reference tables Periodic Table
Rules For Oxidation Numbers 2 2. The oxidation of an atom in its uncombined, elemental form is 0. Metallic elements just written as a metal: K(s), Cu(s), or any of the diatiomics: N2, O2, H2. (Notice that O and H are not in a compound but bonded to themselves so this rule supersedes the following two.)
Rules For Oxidation Numbers 3 3. The oxidation number of hydrogen in a compound is usually +1, especially (but not always) if it is written first in the compound (like H2O, HCl). It will be -1 when bonded to a metal and is written second: (NaH).
Rules For Oxidation Numbers 4 4. For Regents Chemistry you always assume that oxygen s oxidation number, when bonded to something else, is -2 unless specifically told otherwise. The only time it is -1 is in a peroxide: H2O2.
Rules For Oxidation Numbers 5 & 6 5. For any neutral compound, the sum of the oxidation numbers of the atoms in the compound must equal 0. 6. For a polyatomic ion, the sum of the oxidation numbers must equal the charge on the ion. (i.e. use Table E in Reference Tables.) Crack out the mighty and powerful reference tables.
Almost Always Alkali Metals are +1 Alkaline Earths are +2 Halogens are -1 Oxygen is -2 IF there is a polyatomic ion, use the chart in the reference table and figure out what the other ion would be.
Identifying Reactions All chemical reactions can be assigned to one of two classes. #1 Redox - electrons are transferred from one reacting species to another. single-replacement reactions, synthesis reactions decomposition reactions combustion reactions
#2 Other reactions - no electron transfer occurs. double-replacement reactions acid-base reactions