Biochemical Reactions
Exploring the basics of chemical reactions, the conservation of matter principle, exothermic and endothermic reactions, and the role of activation energy in jumpstarting reactions. Learn how elements transform to create products, the significance of energy release or absorption, and the essential co
3 views • 19 slides
Overview of Serious Adverse Reactions and Transfusion Events
This data compilation covers the reporting trends, breakdown of reports, components issued, and specific types of adverse transfusion reactions experienced within the National Healthcare Organization (NHO) from 2019 to 2022. The information includes statistics on Serious Adverse Events (SAE), Seriou
2 views • 46 slides
Chemical Analysis and Redox Reactions in Chemistry
Iron(II) ethanoate concentration determination using redox titration with cerium(IV) sulfate, balanced redox equations for manganate(VII) ion oxidizing iron(II) ion, and calculations of iron percentage in samples using titration with potassium manganate(VII). Molarities and concentrations are calcul
1 views • 6 slides
Overview of Organic Reactions and Mechanisms
Organic reactions can be categorized into addition, elimination, and substitution reactions, occurring through either polar or free radical mechanisms. Polar reactions may be electrophilic or nucleophilic, while free radical reactions involve radicals reacting to complete electron octets. Different
2 views • 26 slides
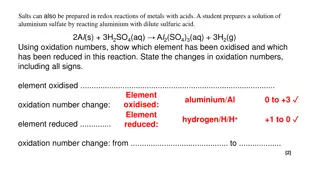
Redox Reactions in Chemistry
Salts can be prepared through redox reactions involving metals and acids. This interactive lesson covers oxidation numbers, identifying oxidized and reduced elements, and explaining electron transfer in redox reactions. Examples include reactions of aluminum with sulfuric acid and magnesium with cop
2 views • 12 slides
Enzyme Kinetics for Understanding Chemical Reactions
Enzyme kinetics is a vital discipline focusing on the rate of enzyme-catalyzed reactions and how they respond to varying conditions. Reactions are classified based on reactant concentration influences. Zero, first, second, and third order reactions are distinguished, with examples like first-order r
0 views • 31 slides
Electrochemical Processes in Materials Engineering
Electrochemical processes play a crucial role in materials engineering, specifically in the context of corrosion. These processes involve both oxidation (anodic reaction) and reduction (cathodic reaction) reactions occurring simultaneously. Maintaining a balance between these reactions is essential
3 views • 22 slides
Enemark-Feltham Notation in Iron-Nitrosyl Complexes
Iron-Nitrosyl complexes are redox non-innocent, with NO exhibiting multiple redox states. Enemark-Feltham Notation helps in determining metal-ligand interactions and oxidation states. Detailed information on NO ligands, bonding characteristics, and methods for analyzing iron-NO systems are discussed
0 views • 6 slides
Energy Changes in Chemical Reactions
Exothermic reactions release energy to the surroundings, exhibited in processes like respiration and combustion. On the other hand, endothermic reactions absorb energy, demonstrated in examples such as photosynthesis. By observing changes in temperature and reactions between various substances, one
0 views • 24 slides
Chemical Reactions and Energy Transfers
Understanding chemical reactions involving thermal decomposition of metal carbonates, identifying exothermic and endothermic reactions based on energy transfers, and recognizing oxidation and reduction in redox reactions.
1 views • 5 slides
Soil Chemistry and Redox Reactions in Environmental Chemistry
Soil chemistry plays a crucial role in sustaining healthy soils by influencing nutrient availability through oxidation and reduction processes. Redox reactions in soil are impacted by factors like oxygen content and water presence, affecting nutrient supplies. The redox status of soil reflects its n
1 views • 92 slides
Redox Titration: Potassium Permanganate with Iron(II) Salt Assay
Ferrous sulfate (FeSO4.7H2O) is used in medical treatments for iron deficiency. This article discusses the redox titration process involving potassium permanganate and ferrous sulfate, along with the chemical principles, procedure, and calculations involved. Potassium permanganate is a powerful oxid
5 views • 7 slides
Mechanisms and Models of Nuclear Reactions
The chapter discusses the reaction cross-section in nuclear reactions, including resonance and tunneling phenomena. It explains the probability of reactions occurring, the influence of nuclear radius on cross-section, and how tunneling allows reactions at energies lower than the Coulomb barrier. Exa
0 views • 22 slides
Nuclear Reactions: A Comprehensive Overview
Nuclear reactions involve direct and compound scenarios, with direct reactions occurring in a short period and compound nucleus reactions leading to long-lived excited states. Different types of reactions like elastic scattering, break-up, and compound nuclear reactions are discussed, highlighting t
5 views • 11 slides
Electrode Reactions in Electrochemistry
Exploring electrode reactions in electrochemistry involves delving into Faraday's law, coulometry, and the importance of sustainable electrode reactions. These concepts help us understand how the quantity of charge passed affects the production or consumption of substances in electrode reactions. As
4 views • 27 slides
Energy Changes in Chemical Reactions
Energy changes in chemical reactions can be categorized as exothermic and endothermic. Exothermic reactions release energy to the surroundings, while endothermic reactions absorb energy from the surroundings. Examples and uses of both types of reactions are provided, along with details on measuring
4 views • 24 slides
Hypersensitivity Reactions and Classification
Hypersensitivity reactions occur in sensitized hosts following contact with specific antigens, leading to injurious consequences. The Gell and Coombs Classification categorizes reactions into Type I, II, III, and IV based on immune response and duration. Type I reactions are immediate and humoral, w
0 views • 30 slides
Redox Reactivity and Balancing Equations in Acidic Solutions
Exploring the concept of reactivity in redox reactions using zinc, nickel, and copper, followed by a detailed guide on balancing redox equations in acidic solutions. Learn how to determine oxidation numbers, identify redox atoms, balance charges, and handle oxygen and hydrogen atoms to achieve balan
0 views • 18 slides
Oxidation-Reduction Reactions in Chemistry
Explore the concept of oxidation and reduction in chemistry, which are fundamental processes that occur simultaneously in oxidation-reduction reactions. Learn about the role of oxygen, different types of oxidation reactions beyond burning, such as bleaching stains, and the concept of reduction invol
0 views • 34 slides
Key Fusion Reactions in Nuclear Astrophysics
Fusion reactions play a crucial role in nuclear astrophysics, with key reactions involving light elements such as Li, Be, B, and stable carbon isotopes. Understanding fusion of light heavy nuclei at extreme energies is essential for predicting stellar evolution. The S-factor provides a convenient re
0 views • 31 slides
Chemical Reaction Kinetics: From Unimolecular to Three-Body Reactions
Explore the fundamental concepts of chemical reactions, including unimolecular reactions like thermolysis and photolysis, bimolecular reactions, and three-body reactions. Learn about rate constants, reaction mechanisms, and the impact of pressure on reaction rates. Discover how energy transfer, phot
0 views • 9 slides
Mineral Reactions in Metamorphism
Mineral reactions play a crucial role in our comprehension of metamorphism, helping to estimate the pressures and temperatures rocks undergo. These reactions can be categorized as continuous or discontinuous, leading to different mineral products. Discontinuous reactions, exemplified by the transfor
0 views • 6 slides
Electron Transport Chain and ATP Synthesis in Biochemistry
This course delves into the intricacies of electron transport and oxidative phosphorylation in biochemistry, elucidating how NADH and FADH2 are re-oxidized to generate ATP in eukaryotes and prokaryotes. It explores redox potential, oxidation-reduction reactions, and the role of standard redox potent
0 views • 20 slides
Chemical Reactions and Catalysts
Chemical reactions involve the formation of new substances from reactants, with key processes like oxidation and reduction. Reversible reactions, endothermic and exothermic reactions, and the role of catalysts in speeding up reactions are explored. The significance of chemical symbols, formulas, and
0 views • 8 slides
Nuclear Reactions: Fission, Fusion, and Energy Release
This content covers various aspects of nuclear reactions, including nuclear fission, fusion reactions, the Manhattan Project, and examples of reactions involving different particles and elements. It explains concepts like exoergic and endoergic reactions, conservation of charge and nucleon number, a
0 views • 34 slides
Electrochemistry Concepts and Redox Reactions
Explore the fundamentals of electrochemistry, oxidation-reduction reactions, and identification of redox components. Learn about oxidation states, electron transfer, and half-reactions. Dive into examples and visual aids to grasp the concepts easily.
0 views • 42 slides
Electrochemistry: Redox Reactions, Cells, and Equations
Electrochemistry is a branch of chemistry that involves redox reactions, galvanic cells, standard reduction potentials, balancing redox equations, batteries, corrosion prevention, and electrolysis. Learn about the fundamental principles, examples of redox reactions, and how to balance equations usin
0 views • 61 slides
Cyclic Voltammetry in Electrochemical Methods
Electrochemical methods, such as cyclic voltammetry, are crucial for studying electron transfer processes, redox reactions, and adsorption on surfaces. Cyclic voltammetry involves varying the applied potential at a working electrode to monitor electron flow and chemical reactions. Peaks in the curre
0 views • 11 slides
Oxidation-Reduction Reactions in Analytical Chemistry
Oxidation-reduction reactions play a crucial role in various chemical processes, including photosynthesis and corrosion. This content delves into the basics of redox reactions, explaining how electrons are transferred between reactants, leading to changes in oxidation numbers. Examples such as the r
0 views • 10 slides
Energy in Chemical Reactions
Chemical reactions involve the release or absorption of energy in various forms like heat, light, sound, and electricity. Exergonic reactions release energy, while endergonic reactions absorb energy. Catalysts speed up reactions, while inhibitors slow them down without changing the amount of reactan
0 views • 8 slides
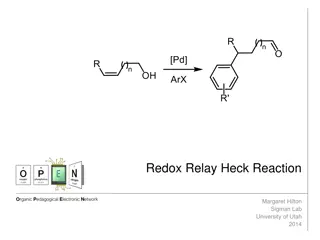
The Redox-Relay Heck Reaction in Organic Synthesis
The Redox-Relay Heck Reaction is a powerful tool in organic synthesis that allows for the functionalization of olefins with aryl groups. Developed by Sigman and colleagues, this reaction involves a palladium-catalyzed relay controlled by a thermodynamic sink, leading to the formation of aldehydes or
0 views • 6 slides
Redox Conditions and pH Control in a Mountain Watershed: Study in Red Canyon, Wyoming, USA
Exploring redox conditions and trace element concentrations in a semi-arid mountain watershed, this study in Red Canyon, Wyoming, delves into the impact of oxic surface water and anoxic groundwater on trace element cycling. The investigation aims to enhance understanding of seasonal variabilities, f
0 views • 11 slides
Electrochemical Systems and Processes
Electrochemical engineering involves the study of devices and processes that convert chemical energy to electrical energy through ionic conductors. This field explores redox reactions, energy-producing processes, electrocatalysis, anodic and cathodic reactions, and the interplay between thermochemic
0 views • 25 slides
Neutron Production Reactions at ILC - Applications and Estimations
Neutron production reactions at the International Linear Collider (ILC) involving nuclear reactions, photo-nuclear reactions with light and heavy nuclei, estimation of neutron production using gamma irradiation on Be-9, and application prospects. Cross-sections, energy inputs, and conversion rates a
0 views • 29 slides
Reactions of Alkenes: Strategies and Mechanisms
The excerpt provides detailed insights into reactions of alkenes, emphasizing main reaction classes, key characteristics, intermediate states, and specific mechanisms like bridgehead halohydrin. It covers important concepts such as carbocation addition, free radical addition, and organometallic/redo
0 views • 12 slides
Assay of Ferrous Sulfate (FeSO4.7H2O) by Redox Titration Experiment
This experiment involves determining the weight and weight percentage of an unknown sample of FeSO4.7H2O through a redox titration using potassium permanganate solution. Ferrous sulfate, a chemical compound used in medical treatments, is oxidized to ferric sulfate in the presence of sulfuric acid. T
0 views • 9 slides
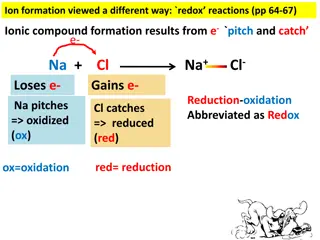
Redox Reactions and Ionic Compound Formation
Explore the concept of redox reactions through the process of ion formation, where elements lose or gain electrons to create ionic compounds. Learn about oxidation (ox) and reduction (red) in chemical reactions, and how to identify which elements lose or gain electrons based on charge changes. Disco
0 views • 14 slides
Redox Half Reactions: Assigning Oxidation Numbers and Half-Reactions
In this content, we explore assigning oxidation numbers to elements in compounds such as HClO, HClO2, HClO3, and HClO4. We then delve into the Haber Process to understand redox reactions. The concept of oxidation and reduction, as well as the significance of electrons in these reactions, is illustra
0 views • 20 slides
Microtester for Low Microbial Contamination Detection
The Microtester method offers rapid, reliable detection of low microbial contamination by measuring redox-potential changes in the growth environment. This innovative approach reduces incubation time and provides concentration-dependent results. Utilizing a MicroTester System with water baths, test
0 views • 34 slides
Lecture 5a
Redox reactions involve the transfer of electrons between chemical species. They play a significant role in various processes such as combustion, batteries, and biological functions. Understanding these reactions is crucial in fields like electrochemistry. Through examples like the oxidation of copp
0 views • 20 slides