NGM282 in NASH: Phase 2 Study on Liver Fat Reduction and Histology Changes
A phase 2 study on NGM282 in NASH patients showed a significant decrease in liver fat content, meeting the primary endpoint. Exploratory findings also indicated potential improvements in liver histology. The treatment involved NGM282 at 3 mg QD, with additional rosuvastatin if needed. Promising results were observed in liver biopsies, MRI-PDFF measurements, and NAFLD activity scores, highlighting the potential of NGM282 in treating NASH. Fibrosis markers also showed positive trends, suggesting a comprehensive impact on liver health.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
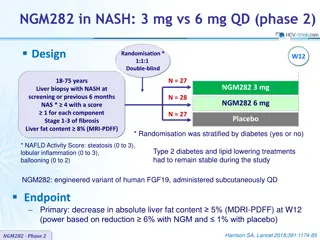
NGM282 in NASH: 3 mg QD (phase 2) Design W2 W12 Liver biopsy with NASH NAS * 4 with a score 1 for each component Stage 1-3 of fibrosis Liver fat content 8% (MRI-PDFF) NGM282 3 mg + rosuvastatin (if needed) NGM282 3 mg N = 19 Screening X X X W6 - X X W12 X X X W18 - X X Assessments Liver Biopsy MDRI-PDFF LiverMultiScan * NAFLD Activity Score: steatosis (0 to 3), lobular inflammation (0 to 3), ballooning (0 to 2) NGM282: engineered variant of human FGF19, administered subcutaneously qd Rosuvastatin: started at W2 if LDL-cholesterol rise of 10 mg/dl observed Endpoints Primary: decrease in absolute liver fat content 5% at W12 Exploratory: change in liver histology at W12 Harrison SA, EASL 2018, Abs. GS-014 NGM282-Phase 2
NGM282 in NASH: 3 mg QD (phase 2) Baseline characteristics, mean or N or % NGM282 3 mg N = 19 Age, years (SD) 51.4 (12.6) Male / Female, N 4/15 Diabetes mellitus, N 7/12 Liver fat content, MRI-PDFF (proton density fat fraction), % (SD) 17.1% (5.6) AST, U/l (SD) 65 (32) ALT, U/l (SD) 82 (39) Fibrosis stage (SD) 2.5 (0.8) NAFLD activity score (SD) 5.7 (1.5) Liver-Inflammation-Fibrosis (LIF) score (SD) 2.80 (0.43) LDL cholesterol, mg/dL (SD) 97 (27) Statin na ve/experienced 13/6 7 -hydroxyl-4-cholesten-3-one (C4), ng/ml (SD) 35.1 (24.2) Harrison SA, EASL 2018, Abs. GS-014 NGM282-Phase 2
NGM282 in NASH: 3 mg QD (phase 2) Effect on FGF19 targets (N = 19) Mean C4 levels, ng/mL Mean Total serum bile acids, mol/L p < 0.0001 p < 0.0001 40 6 p < 0.0001 p < 0.0001 35 5 at W12 Mean (SD) at W12 Mean (SD) 30 -33.2 (24.4) -3.5 (2.5) Absolute Absolute 4 25 -93% (10) -64% (41) Relative Relative 3 20 15 2 10 1 5 0 0 Baseline W6 W12 Baseline W6 W12 C4 = 7 -hydroxyl-4-cholesten-3-one Lower limit of C4 quantitation = 0.9 ng/ml Harrison SA, EASL 2018, Abs. GS-014 NGM282-Phase 2
NGM282 in NASH: 3 mg QD (phase 2) Primary endpoint: mean % absolute change in liver fat content at W12 (N = 19) p < 0.0001 20 p < 0.0001 at W12 Mean (SD) 15 -11.2% (4.2) -67% (17) Absolute Relative 10 5 0 Baseline W6 W12 100% of subjects achieved primary endpoint of 5% absolute liver fat content reduction and a decreased of relative liver fat content 30% at W12 12 (63%) subjects normalized liver fat content ( 5%) by W12 Harrison SA, EASL 2018, Abs. GS-014 NGM282-Phase 2
NGM282 in NASH: 3 mg QD (phase 2) Fibrosis markers changes at W12, N = 19 Mean PRO-C3 levels (ng/mL) ELF Score and components p < 0.05 6 p < 0.01 Mean (SD) Baseline W6 W12 10.1 (1.0) 9.6* (0.8) 9.5* (1.0) ELF score 5 at W12 Mean (SD) 91.4 (79.7) 67.3 (60.2) 72.1 (90.3) -11.1 (18.4) Absolute Hyaluronic acid 4 -33% (47) Relative 13.4 (4.6) 9.7* (3.5) 10.3* (3.4) PIIINP 3 270.8 (67.7) 233.6* (51.4) 232.0* (62.5) TIMP-1 2 *p < 0.01 1 Subjects with severe disease (ELF 10.1) decreased ELF score by 0.8 at W12 0 Baseline W6 W12 Harrison SA, EASL 2018, Abs. GS-014 NGM282-Phase 2
NGM282 in NASH: 3 mg QD (phase 2) NAS histological response at W12, N = 19 % NAS Steatosis Inflammation Ballooning 100 90 80 42% 53% Improved 70 74% 60 84% 50 40 53% 30 42% No change 20 26% 11% 5% 10 0% Worsened 5% 5% 0 Mean change at W12 (SD) -2.3 (1.8) -1.1 (0.9) -0.5 (0.8) -0.7 (0.9) Harrison SA, EASL 2018, Abs. GS-014 NGM282-Phase 2
NGM282 in NASH: 3 mg QD (phase 2) Fibrosis evolution at W12 (liver biopsy), N = 19 42% Improved 47% No change 11% Worsened Mean change from baseline in fibrosis stage: - 0.5 3 subjects had a 2-stage improvement in fibrosis: all F3 changed for F1 Mean decrease in NAS in subjects with improved fibrosis: -3.5 Harrison SA, EASL 2018, Abs. GS-014 NGM282-Phase 2
NGM282 in NASH: 3 mg QD (phase 2) Mean transaminases (U/L), N = 19 100 at W12 ALT AST 90 ALT Absolute Relative -53* -60%* -37* -52%* 80 70 60 *p < 0.0001 50 AST 40 30 20 10 0 Baseline W2 W4 W6 W8 W10 W12 Harrison SA, EASL 2018, Abs. GS-014 NGM282-Phase 2
NGM282 in NASH: 3 mg QD (phase 2) LDL-cholesterol (mg/dL) changes during treatment NGM282 3 mg NGM282 3 mg Rosuvastatin (if needed) 200 150 100 50 LDL-C levels return to baseline and/or target of < 100 mg/dL with statin treatment 0 Baseline W2 W4 W6 W8 W10 W12 Decreased C4 and increased LDL-cholesterol reflect potent CYP7A1 inhibition Lipid particle change primarily driven by increase in large LDL particles Significant reductions in serum triglycerides (56%) and no change in HDL Harrison SA, EASL 2018, Abs. GS-014 NGM282-Phase 2
NGM282 in NASH: 3 mg QD (phase 2) Safety and tolerability Favorable safety and tolerability profile consistent with other NGM282 studies (no new safety signals identified) Mild gastrointestinal symptoms (loose/frequent stools) remain the most common treatment emergent adverse events Majority were mild and resolved during treatment phase No subject withdrew from treatment due to any drug-related adverse events Gastrointestinal symptoms were largely mitigated with separating the timing of injection around meals and decreasing meal size Three severe adverse events, all unrelated to study drug: Pleurisy Chest tightness Cardiac arrest (non myocardial infarction) Harrison SA, EASL 2018, Abs. GS-014 NGM282-Phase 2
NGM282 in NASH: 3 mg QD (phase 2) Summary Potent C4 and bile acid suppression consistent with FGF19 hormone activity Significant and clinically meaningful reductions across non-invasive markers of NASH-related disease Large percentage of patients demonstrated histological improvement at W12 Statin co-administration rapidly mitigates LDL-cholesterol elevations Treatment was safe and well tolerated Harrison SA, EASL 2018, Abs. GS-014 NGM282-Phase 2