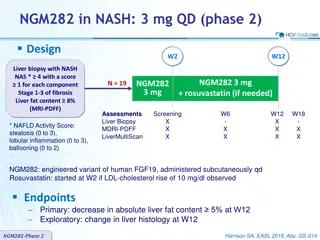
NGM282 in NASH: Phase 2 Study on Liver Fat Reduction and Histology Changes
A phase 2 study on NGM282 in NASH patients showed a significant decrease in liver fat content, meeting the primary endpoint. Exploratory findings also indicated potential improvements in liver histology. The treatment involved NGM282 at 3 mg QD, with additional rosuvastatin if needed. Promising resu
0 views • 11 slides
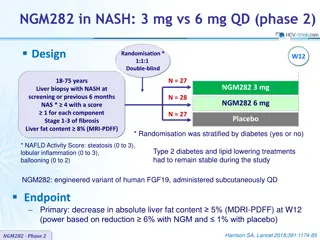
NGM282 in NASH Phase 2: 3 mg vs 6 mg QD Randomised Trial
This Phase 2 clinical trial compares the efficacy of NGM282 (3 mg vs 6 mg) versus placebo in patients with NASH. The primary endpoint is a 5% reduction in liver fat content at 12 weeks. Randomisation was stratified by diabetes status, and stable Type 2 diabetes and lipid-lowering treatments were mai
0 views • 6 slides