Antigen Testing in Higher Education Institutions
Antigen testing strategies are recommended for institutions of higher education, with rapid results provided by CareStart COVID-19 Antigen Tests. The testing procedure involves sample collection and interpretation of results, including criteria for validity and different outcomes. Regular testing cadences and robust testing strategies are advised, particularly in high-risk circumstances. CareStart testing instructions outline steps for administering the test effectively.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
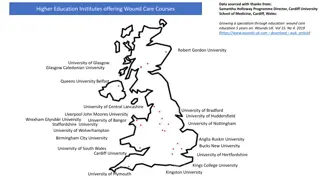
Institutions of Higher Education Antigen Testing
Testing Strategies in Institutions of Higher Education MDHHS has a supply of antigen tests available to institutions of higher education The CDC has released guidance as it relates to testing strategies on campus MDHHS recommends an ongoing testing cadence Example: Weekly testing in a small campus or dorms or weekly testing of a subset of students for a larger campus Additional testing in high-risk circumstances Outbreaks, contact sports, returning travelers, exposure, detection of a variant of concern, symptomatic individuals
CareStart COVID-19 Antigen Tests Rapid results within 10 minutes No additional lab equipment or instruments required Nasal swab specimen collection CLIA waived test
Test Procedure 4 2 1 3 5 Invert the extraction vial and hold the sample vertically above the sample well. Squeeze the vial gently. Allow three (3) drops of sample to fall into the sample well. Close the vial by pushing the cap firmly onto the vial and mix thoroughly by flicking the bottom of the tube. Remove the swab by rotating against the extraction vial while squeezing the sides of the vial to release the liquid from the swab. Discard the swab in biohazard waste. Peel off aluminum foil seal and rotate the swab inside the extraction vial vigorously at least 5 times Read the results at 10 minutes. The test results should not be read after 15 minutes.
Result Interpretation Invalid COVID-19 Positive COVID-19 Negative If the red-colored line in the Control region C is not visible, the result is invalid (even if Test T line is visible) Repeat test using remaining sample in the extraction vial with a new test device (within 4 hours of addition to extraction buffer) Red Control Line C visible Blue Test Line T visible Any faint colored line in the test region is considered positive Red Control Line C must be visible No Test Line T (blue) visible
CareStart Testing How-To Steps to Administer CareStart Antigen Test Step Step 1 1 Step Step 2 2 Step Step 3 3 Step Step 4 4 Set up test area Allow all materials to equilibrate to room temperature Spread a paper towel to use as a work surface Ready timing device Ready Biohazard bag Set up test Set up disposable elution tube rack Don PPE Mark X with sharpie at an unused location and place new elution tube Only open ONE test at a time Mark elution tube with patient identifier (sharpie) Remove the aluminum foil seal from the elution tube Administer Test Remove nasal swab from pouch Insert the swab into patient s left or right nostril Rotate the swab there for 10 seconds Repeat in other nostril with same swab Place swab in elution vial and rotate vigorously 5+ times Prepare sample, place in well Place swab in elution vial and rotate vigorously 5+ times Remove the swab by squeezing the side of the vial so it rubs against the vial walls Discard the swab in the biohazard bag Close the vial by clicking the cap into place Shake thoroughly and flick bottom of tube to mix Squeeze 3 drops of solution from the vial into the sample well Time 10 minutes Step Step 5 5 Interpret test* POSITIVE Test Included in Kit Included in Kit Not Included in Kit Not Included in Kit NEGATIVE Test INVALID Test Nasal Swab COVID-19 Antigen Extraction (elution) Vial Extraction (elution) Vial Caps COVID-19 Antigen Test Device Disposable elution tube rack But needed: PPE Disinfectant effective against COVID-19 Biohazard bags Timer (do not use personal cell phone) *Results can NOT be interpreted after 15 minutes
Steps to set up a testing program 1 2 3 4 5 Plan for specimen collection Plan for positive tests Amend or apply for a CLIA waiver Results reporting Trainstaff
Step 1: Amend/Apply for a CLIA Amend: Send an email to BCHS-CLIA@michigan.gov Body needs to include: Site address, CLIA #, and state that you want to add the Abbott BinaxNOW COVID-19 Ag Card, BD Veritor Systems for Rapid Detection of SARS-CoV-2, CareStart COVID-19 Antigen Test to be added to the CLIA waiver. Apply: To receive a CLIA waiver, facilities should complete the CLIA waiver application and submit it to LARA-BCHS-DHHS-COW-TESTING-APPLICATION@michigan.gov. No specific credentials are required to obtain a CLIA waiver. The site performing the testing must follow the guidelines specified under the waiver. The cost is $180 for two years.
Step 2: Train Staff Attend MDHHS office Attend MDHHS office hours with questions hours with questions View the online training View the online training video video here Identify testing team Identify testing team Watch the recording of Watch the recording of the live MDHHS the live MDHHS training sessions training sessions here here - Staff to perform tests - Staff to complete result reporting
Step 3: Plan for Specimen Collection Ensure consent is obtained from each participant! Supplies needed for Antigen Testing PPE (CDC GUIDELINES) Masks Gloves Eye protection: Face shield or goggles Hand sanitizer Disinfectant Pens/Permanent Markers Timer Reporting Portal WIFI & Laptop Have a plan for waste disposal
Step 4: Plan for testing positive Symptomatic (first seven days) or close contact/known exposure Asymptomatic and no close contact Presumptive COVID-19 case Prompt isolation Confirm with a PCR test* COVID-19 case Positive Result Prompt isolation Negative No additional follow-up necessary Reinforce prevention measures Presume negative An individual who is a close contact/known exposure must still complete a 14-day quarantine Negative Result Confirm result with a PCR test*
Step 5: Antigen Testing Results Facility Info Select School Enter EEM CODE EEM CODE School type In the facility name please write [SCHOOL NAME] Entity Type Ex., Michigan State Students Individual Info ID # ID # Name/Address/Info Results Ex., Michigan State Staff This reporting must be completed on the day of reporting Antigen Testing Results Link
Questions? Interested schools should enroll here