Understanding Stoichiometry and Solution Chemistry in Toxins
Exploring how to determine the count of very small objects using mass measurements, especially in chemical compounds like arsenic oxide. The discussion includes calculating lethal doses, finding average mass, and utilizing percent error to express measurement accuracy in chemistry applications.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Living By Chemistry SECOND EDITION Unit 4: TOXINS Stoichiometry, Solution Chemistry, and Acids and Bases
Lesson 75: Make It Count Counting By Weighing
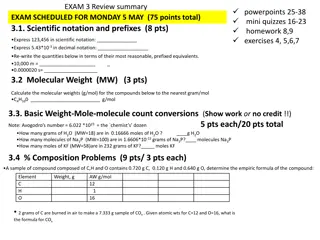
ChemCatalyst The LD50of arsenic (III) oxide, As2O3, is 15 mg/kg. a) Figure out the lethal dose for a 150 lb adult. b) How many atoms do you think are in a lethal dose of arsenic (III) As2O3? What would you need to know in order to find out?
Key Question How can mass help you count large numbers of small objects?
You will be able to: explain how large numbers of small objects are determined calculate the percent error of a calculation
Prepare for the Activity Work in groups of four.
Discussion Notes The easiest way to determine the count of very small objects is to find their total mass and divide by the mass of one object. In order to get a more accurate average mass measurement for a tiny object, it is better to find the mass of 10 or 20 of the objects and divide by the number of objects to find the average mass, especially if the variation in size is slight.
Discussion Notes (cont.) Chemists use mass when measuring chemical compounds because it is not possible to count atoms directly.
Discussion Notes (cont.) Chemists use percent error to express how close their measurements are to the accepted value. Percent error = |observed value actual value| 100 actual value
Discussion Notes (cont.) Object Mass (g) Object Mass (g) seed bead 0.0056 g large pony bead 0.26 g #18 medium rubber band sequin 0.0085 g 0.27 g grain of rice 0.022 g elbow pasta 0.287 g tiny rubber band 0.048 g large sticky note 0.40 g small pony bead 0.067 g plastic paper clip 0.45 g split pea 0.090 g kidney bean 0.55 g #33 large rubber band #10 small rubber band 0.126 g 0.57 g small sticky note 0.15 g business card 0.80 g small metal paper clip #8 lock washer 0.136 g 0.94 g
Wrap Up How can mass help you count large numbers of small objects? It is possible to count large numbers of small objects by weighing them together and dividing by the mass of a single object. It is more accurate to weigh a large sample of a collection of objects and find their average mass than to rely on weighing a single object. There are so many atoms in a sample that you cannot count them. Chemists use mass to calculate numbers of atoms.
Check-In You have a sandwich bag containing raisins. It weighs 24.6 g. A sample of ten raisins weighs 0.90 g. The empty bag has a mass of 2.90 g. How many raisins are in your sandwich bag?