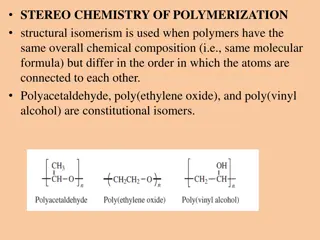
Understanding Polymers: Monomers to Polymers
Explore the world of polymers by learning about monomers and polymers, how alkenes are used to create polymers, and the process of polymerisation. Dive into the structure of poly(ethene) and poly(propene) through diagrams and hands-on activities with molymod kits.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
What does poly mean? What does mono mean? Monorail Monobrow
Objectives Define the terms monomer and polymer Describe how alkenes are used to make polymers Draw the repeat unit of addition polymers, including poly(ethene) and poly(propene) Deduce the structure of a monomer from the repeat unit of an addition polymer
Molymod kits Using the molymod kits make an ethene molecule (C2H4) in pairs Once you have made one, join up with another group and join your two ethene molecules together What had to happen for them to join?
Monomers and polymers Ethene is a monomer (a small reactive molecule subunit used to create large molecules known as polymers) Ethene monomers join together to create poly(ethene) in a process known as polymerisation. To allow the monomers to join, the carbon double bonds are opened up and replaced by single bonds.
POLYMERISATION So what is a POLYMER? Monomers Polymers are two or more . bonded together!
To help you remember: To help you remember: What do you call hundreds and thousands of Olly Murs forming a very long chain? POLYMERS
Polymerisation Ethene monomers Poly(ethene) Repeating unit of poly(ethene) Copy the diagram and describe what happens in this reaction using the words in the box below n is the number of monomers joined together monomer polymer polymerisation ethene poly(ethene) carbon double bond
1 Draw the polymer that was produced from this alkene.
Did you get it?
Same elements in same places No double bond Lines outside of the brackets n on right hand side
2 Draw the polymer that was produced from this alkene
Did you get it?
3 What is the monomer used to make the polymer called poly(but-1-ene)
Poly(butene) Polymeans polymer The word in the bracket is the SAME as the monomer from which is was made
4 Draw the monomer used to make the polymer called poly(2-methylprop- 1-ene)
Uses of polymers Many polymers are plastics. Poly(ethene) is very useful as it is easy to shape, strong and transparent (unless we add colouring to it). Plastic bags, plastic drinks bottles, dustbins and clingfilm are all examples of poly(ethene) aka polythene. Poly(propene) forms a strong, tough plastic which is used to make many things including carpets, milk crates and ropes.
Polymers form different structures: Separate polymer chains with no bonds/ weak intermolecular forces between them results in a flexible plastic so the plastic can be melted and reformed Separate polymer chains with bonds between them means the chains can t separate or slide past each other which is more rigid
Task GROUP A S focus on new and useful polymers Your job is to create a summary poster/mind map detailing uses of newly developed polymers. GROUP B S focus on plastic waste Your job is to create a summary poster/mind map detailing the problems associated with disposing of plastics and how these problems can be solved. You will be explaining to other groups what you have found out!
Objectives Define the terms monomer and polymer Describe how alkenes are used to make polymers Draw the repeat unit of addition polymers, including poly(ethene) and poly(propene) Deduce the structure of a monomer from the repeat unit of an addition polymer