Stereochemistry and Isomerism in Polymerization
Structural isomerism plays a crucial role in polymer chemistry by distinguishing polymers with the same molecular formula but different atom connectivity. Isomeric polymers can stem from different monomers or polymerization routes, resulting in variations in properties. Stereoisomerism, on the other hand, involves configurations and spatial orientations of atoms in polymers, leading to different structures such as cis-trans isomers and tacticity variations like isotactic and syndiotactic arrangements.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
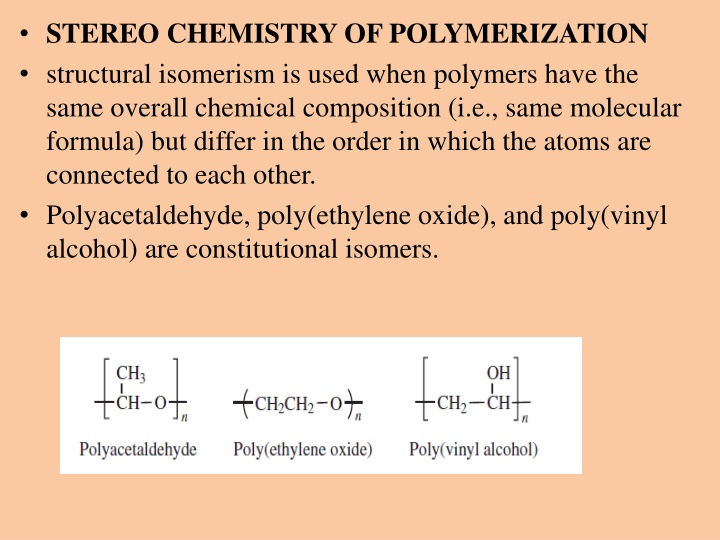
STEREO CHEMISTRY OF POLYMERIZATION structural isomerism is used when polymers have the same overall chemical composition (i.e., same molecular formula) but differ in the order in which the atoms are connected to each other. Polyacetaldehyde, poly(ethylene oxide), and poly(vinyl alcohol) are constitutional isomers.
Poly(methyl methacrylate) and poly(ethyl acrylate) are isomeric polymers formed from isomeric monomers Poly( -caprolactam) and poly(hexamethylene adipamide) are isomeric even though the monomers are not isomeric.
Isomeric polymers can also be obtained from a single monomer if there is more than one polymerization route. The head-to-head placement that can occur in the polymerization of a vinyl monomer is isomeric with the normal head-to-tail placement
Monomers with two polymerizable groups can yield isomeric polymers if one or the other of the two alternate polymerization routes is favored. Examples of this type of isomerism are the 1,2- and 1,4-polymers from 1,3-dienes The isomeric polymers usually differ considerably in their properties.
TYPES OF STEREOISOMERISM IN POLYMERS Stereoisomers have the same connectivity, but differ in their configurations. Configuration is the relative orientation in space of the atoms of a stereoisomer. Cis trans (geometric) isomers arise from different configurations of substituents on a double bond or on a cyclic structure. The term configuration should not be confused with conformation. Conformation refers to the different orientations of atoms and substituents in a molecule that result from rotations around single bonds.
Monosubstituted Ethylenes Tacticity The regularity in the configurations of successive stereocenters determines the overall order (tacticity) of the polymer chain. If the R groups on successive stereocenters are randomly distributed on the two sides of the planar zigzag polymer chain, the polymer does not have order and is termed atactic. Two types of ordered or tactic structures or placements can occur: isotactic and syndiotactic. An isotactic structure occurs when the stereocenter in each repeating unit in the polymer chain has the same configuration. All the R groups will be located on one side of the plane of the carbon carbon polymer chain either all above or all below the plane of the chain.
A syndiotactic polymer structure occurs when the configurations of the stereocenters alternate from one repeating unit to the next with the R groups located alternately on the opposite sides of the plane of the polymer chain. Polymerizations that yield tactic structures (either isotactic or syndiotactic) are termed stereoselective polymerizations (stereospecific polymerization)
Different polymer structures from a monosubstituted ethylene, ( CH2CHR)n .
Disubstituted Ethylenes Disubstituted Ethylenes For disubstituted ethylenes, the presence and type of tacticity depends on the positions of substitution and the identity of the substituents. In the polymerization of a 1,1-disubstituted ethylene, CH2-CRR', stereoisomerism does not exist if the R and R' groups are the same (e.g., isobutylene and vinylidene chloride).
When R and R' are different (e.g., -CH3 and - COOCH3 in methyl methacrylate), stereoisomerism occurs exactly as in the case of a monosubstituted ethylene. The methyl groups can be located all above or all below the plane of the polymer chain (isotactic), alternately above and below (syndiotactic), or randomly (atactic).
1,2-Disubstituted Ethylenes The polymerization of 1,2-disubstituted ethylenes, RCH- CHR', such as 2-pentene (R=-CH3, R' =-C2H5), presents a different situation. Polymerization yields a polymer structure II in which there are two different stereocenters in each repeating unit. Several possibilities of ditacticity exist that involve different combinations of tacticity for the two stereocenters. Various stereoregular structures can be defined
Diisotactic structures occur when placement at each of the two stereocenters is isotactic. A disyndiotactic structure occurs when placement at each of the two different stereocenters is syndiotactic. Two diisotactic structures are possible. These are differentiated by the prefixes threo and erythro.The erythro structure is the one in which like groups on adjacent carbons are anti to each other (i.e., R and R' are anti to each other). The threo structure has an anti arrangement of unlike groups on adjacent crabons (i.e., R and H are anti and R' and H are also anti). The difference between the erythro and threodiisotactic structures can also be shown by Newman representations of the eclipsed conformation of two consecutive carbon atoms in the polymer chain.
Stereoregular polymers from a 1,2-disubstituted ethylene, (CHR-CHR')n .
1,3-Butadiene and 2-Substituted 1,3-Butadienes 1,2- and 3,4-Polymerizations The polymerization of a 1,3-diene such as 1,3-butadiene, isoprene, and chloroprene can lead to cis and trans isomers when polymerization occurs by a 1,4-polymerization. 1,2-polymerization through one or the other double bond can yield isotactic, syndiotactic, or atactic polymers, for example, for 1,3- butadiene The situation is exactly analogous to the polymerization of monosubstituted alkenes (R=CH=CH2)
With chloroprene and isoprene, the possibilities are enlarged since the two double bonds are substituted differently. Polymerizations through the 1,2- and 3,4-double bonds do not yield the same product as they would in 1,3-butadiene polymerization. There are a total of six structures possible corresponding to isotactic, syndiotactic, and atactic structures for both 1,2- and 3,4- polymerizations for instance, for isoprene, exhibiting the structures with R=-CH=CH2 and -C(CH3)=CH2.
1,4-Polymerization The more important products from 1,3-dienes are those that occur by 1,4- polymerization of the conjugated diene system, for example, for isoprene, 1,4- Polymerization leads to a polymer structure with a repeating alkene double bond in the polymer chain. The double bond in each repeating unit of the polymer chain is a site of steric isomerism since it can have either a cis or a trans configuration. The polymer chain segments on each carbon atom of the double bond are located on the same side of the double bond in the cis configuration (VIII) and on opposite sides in the trans configuration (IX), for example, for polyisoprene. When all of the double bonds in the polymer molecule have the same configuration, the result is two different ordered polymer structures transtactic and cistactic.
Cis and trans 1,4-polymers from isoprene, [CH2CH(CH3)-CHCH2]n .
1-Substituted and 1,4-Disubstituted 1,3-Butadienes 1,2- and 3,4-Polymerizations The polymerizations of 1-substituted (X) and 1,4-disubstituted (XI) 1,3-butadienes involve several possibilities. 3,4-Polymerization of a 1-substituted 1,3-butadiene proceeds with the same possibilities as the polymerization of a monosubstituted ethylene such as propene. 1,2-Polymerization of a 1-substituted 1,3-butadiene as well as 1,2- and 3,4-polymerizations of a 1,4-disubstituted 1,3-butadiene proceed with the same possibilities as 1,2-disubstituted ethylene.
1,4-Polymerization The 1,4-polymerization of a 1-substituted 1,3-butadiene can yield four stereoregular polymer structures. The double bond can have cis or trans configurations, and each of these can be combined with isotactic or syndotactic placement of R groups. The 1,4-polymerization of 1,4-disubstituted 1,3-butadienes leads to structure (XIII), which can exhibit tritacticity since the repeating unit contains three sites of steric
Two of the stereoregular forms of 1,4-poly(1,3-pentadiene), [CH2CH=CHCH(CH3)]n .
ZIEGLERNATTA POLYMERIZATION OF NONPOLAR ALKENE MONOMERS Since the original discoveries of Ziegler and Natta, there have been many hundreds of different combinations of transition and group I III metal components, often together with an added electron donor, studied for use in alkene polymerizations. The major interest will be on the titanium aluminum systems for isoselective polymerization, more specifically, TiCl3 with Al(C2H5)2Cl and TiCl4 with Al(C2H5)3 probably the most widely studied systems, The original initiator used by Ziegler for ethylene polymerization was obtained in situ as a precipitate on mixing the components TiCl4 and Al(C2H5)3 in a hydrocarbon solvent. The mixture was used directly for initiating polymerization. There was a dramatic increase in stereoselectivity when the -, -, or -crystalline form of TiCl3 was used directly.
Most ZieglerNatta components participate in a complex set of reactions involving alkylation and reduction of the transition-metal component by the group I III component as shown below for TiCl4 + AlR3: The mechanism for the stereoselective polymerization of -olefins and other nonpolar alkenes is a -complexation of monomer and transition metal (utilizing the latter s d-orbitals) followed by a four- center anionic coordination insertion process in which monomer is inserted into a metal carbon bond.
Transition-Metal Component The most widely studied transition metal is titanium. At various times, all oxidation states of titanium (II, III, IV) have been proposed for the active site of titanium-based initiators. Most of the evidence points to titanium (III) as the most stereoselective oxidation state, although not necessarily the most active nor the only one. Initiators based on the -, -, and -titanium trihalides are much more stereoselective (isoselective) than those based on the tetrahalide or dihalide. The overall stereoselectivity is usually , , -TiCl3 > TiCl2 > TiCl4 - TiCl3. Changes in the ligands of the transition metal component affect stereoselectivity. For propene polymerization by titanium compounds in combination with triethylaluminum, isotacticity follows the orders
Changes in the transition metal itself have a large effect. Titanium compounds are the most isoselective, while vanadium compounds are the most syndioselective. Differences in initiator stereoselectivity and activity are greatest for the more stereoselective and active transition metal components (trivalent oxidation state).
Group IIII Metal Component The group I III metal component is required to obtain high stereoselectivity and high activity. Aluminum compounds are by far the most often used, a consequence of their ready availability and ease of handling For initiators in which the group I III metal is unchanged, isoselectivity generally decreases as the size of the organic group increases When titanium is the transition metal, the replacement of an alkyl group in AlR3 by any halogen other than fluorine results in increased stereoselectivity and decreased activity Third Component: Electron Donor (Lewis Base) A variety of different compounds have been added to Ziegler Natta initiators to enhance stereoselectivity and activity . These are electron donors (Lewis bases) and include oxygen, water, inorganic and organic halides, phenols, ethers, esters, amines, phosphines, aromatics, carbon disulfide
Mechanism of Isoselective Propagation Structure XX is generally considered as the active species formed from titanium chloride and alkylaluminum components. The in the structure represents an unoccupied (vacant) site of the octahedral titanium complex. The proposed mechanism for isoselective propagation includes monomer coordinates at the vacant site of titanium, resulting in a four-center transition state and subsequent insertion of monomer into the polymer transition metal bond. Polymerization occurs at active sites found on the edges (surfaces) of elementary sheets of the crystal and not in the basal planes.
Mechanism for catalyst site control model of isoselective polymerization.
Primary versus Secondary Insertion; Regioselectivity The insertion reaction shown in Fig.is primary insertion (or 1,2- addition) the unsubstituted end of the double bond carries the partial negative charge and is attached to the counterion G. The other possibility is second insertion (or 2,1-addition) where the substituted end of the double bond becomes attached to G. The two modes of insertion are described by The group I III metal component increases activity and stereoselectivity by alkylation of the transition metal component to form more active and stereoselective reaction sites. The group I III metal component may also be involved in stabilizing the active transition metal sites by complexation and may be part of the counterion structure.
Mechanism of Syndioselective Propagation The synthesis of syndiotactic polymers by Ziegler Natta initiators has been successful for propone, styrene, and some 1,3-dienes
Hindrance between the methyl group of the last unit of the propagating chain and the ligand(s) attached to vanadium prevent rotation about the transition metal carbon bond. This brings into play the repulsive interaction between methyl groups of the terminal monomer unit and incoming monomer. Syndiotactic placement is energetically favored as methyl methyl interactions force the monomer to be coordinated at its opposite face at each successive propagation step. On the other hand, monomer monomer interactions are minimized with isoselective initiators by rotation about the transition metal carbon bond. Isotactic placement occurs since only one configuration is favored for coordination and addition of monomer to the propagating chain. Isotactic placement proceeds with migration of the polymer chain to its original ligand position prior to the next propagation step. Syndiotactic propagation occurs alternately at the two ligand positions.
Termination Propagating chains undergo a number of chain breaking reactions: -Hydride transfer either directly to the transition metal where Ti represents the transition metal active site at which propagation occurs. or to monomer Both -hydride transfers result in polypropene molecules with one vinylidene and one n-propyl end group. The two transfers are zero- and first-order, respectively, in monomer. -Hydride transfer yields vinyl end groups in ethylene polymerization.
2- Chain transfer to the group IIII metal alkyl: Hydrolytic workup of the polymer cleaves the aluminium polymer bond to yield an isopropyl end group. 3- Chain transfer to an active hydrogen compound such as molecular hydrogen: The -hydride transfer reactions produce polymers with one saturated and one unsaturated end group. The other two transfers result in polymers with two saturated groups. The chain-breaking reactions limit the polymer molecular weight, but like typical chaintransfer reactions, they proceed with the reinitiation of new propagating chains.
Rate and Degree of Polymerization The polymerization rate is expressed as where [C*] is the concentration of active sites expressed in moles per liter.The fraction A and M of the transition metal sites covered with the group I III metal component and monomer, respectively, are given by where [A] and [M] are the concentrations of the group I III metal component and monomer in solution, respectively, and KA and KM are the respective equilibrium constants for their adsorption
Propagation occurs by reaction of adsorbed monomer at the active sites at a rate given by Combination of these equations yields the polymerization rate as If the group I III metal component does not compete with monomer for the active sites, thenKA[A] = 0 and the Eq. reduces to
The degree of polymerization is obtained by dividing the propagation rate by the sum of all chain-breaking (transfer) reactions. The degree of polymerization is