Understanding Polymers: Types and Properties
Polymers are large molecules composed of repeated subunits, playing essential roles in everyday life. They can be natural or synthetic, with unique physical properties like toughness and viscoelasticity. Polymers are classified into thermoplastics, thermosets, and elastomers, each with distinct characteristics. Thermoplastics soften when heated and can be reshaped, while thermosets solidify irreversibly and maintain rigidity. This article explores the diverse world of polymers and their applications.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
Apolymer is a large molecule, or macromolecule, composed of many repeated subunits. Due to their broad range of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function.
Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, viscoelasticity, and a tendency to form glasses and semicrystalline structures rather than crystals. The terms polymer and resin are often synonymous with plastic.
The most common way of classifying polymers is to separate them into three groups - thermoplastics, thermosets, and elastomers. The thermoplastics can be divided into two types - those that are crystalline and those that are amorphous.
Molecules in a thermoplastic are held together by relatively weak intermolecular forces so that the material softens when exposed to heat and then returns to its original condition when cooled. Thermoplastic polymers can be repeatedly softened by heating and then solidified by cooling - a process similar to the repeated melting and cooling of metals
Most linear and slightly branched polymers are thermoplastic. All the major thermoplastics are produced by chain polymerization. Thermoplastics have a wide range of applications because they can be formed and reformed in so many shapes. Some examples are food packaging, insulation, automobile bumpers, and credit cards.
A thermosetting plastic, or thermoset, solidifies or "sets" irreversibly when heated; they cannot be reshaped by heating. Thermosets usually are three- dimensional networked polymers in which there is a high degree of cross-linking between polymer chains. The cross-linking restricts the motion of the chains and leads to a rigid material.
Thermosets are strong and durable. They primarily are used in automobiles and construction. They also are used to make toys, varnishes, boat hulls, and glues.
Elastomers are rubbery polymers that can be stretched easily to several times their unstretched length and which rapidly return to their original dimensions when the applied stress is released. Elastomers are cross-linked, but have a low cross- link density.
The polymer chains still have some freedom to move, but are prevented from permanently moving relative to each other by the cross-links. To stretch, the polymer chains must not be part of a rigid solid - either a glass or a crystal. An elastomer must be above its glass transition temperature, TgTg, and have a low degree of crystallinity. Rubber bands and other elastics are made of elastomers.
The attractive forces between polymer chains play a large part in determining polymer's properties. Because polymer chains are so long, these interchain forces are amplified far beyond the attractions between conventional molecules. Different side groups on the polymer can lend the polymer to ionic bonding or hydrogen bonding between its own chains. These stronger forces typically result in higher tensile strength and higher crystalline melting points.
The intermolecular forces in polymers can be affected by dipoles in the monomer units. Polymers containing amide or carbonyl groups can form hydrogen bonds between adjacent chains; the partially positively charged hydrogen atoms in N-H groups of one chain are strongly attracted to the partially negatively charged oxygen atoms in C=O groups on another. These strong hydrogen bonds, for example, result in the high tensile strength and melting point of polymers containing urethane or urea linkages.
Polyesters have dipole-dipole bonding between the oxygen atoms in C=O groups and the hydrogen atoms in H-C groups. Dipole bonding is not as strong as hydrogen bonding, so a polyester's melting point and strength are lower than Kevlar's (Twaron), but polyesters have greater flexibility. Ethene, however, has no permanent dipole. The attractive forces between polyethylene chains arise from weak Van der Waals forces. Molecules can be thought of as being surrounded by a cloud of negative electrons. As two polymer chains approach, their electron clouds repel one another.
STEP GROWTH - SLOW Can use statistical methods as well as kinetics to describe mol. wt. distributions - more on this later CHAIN Polymerization - FAST Can apply statistical methods to an analysis of the microstructure of the products, but not the polymerization process and things like mol .wt.
The reactivity of a functional group is independent of the length of the. chain to which it is attached. A -A + B - B A- AB B Kinetic equation for this type of reaction is usually of the form: Reaction Rate = - d[A] dt = k2 [A][B]
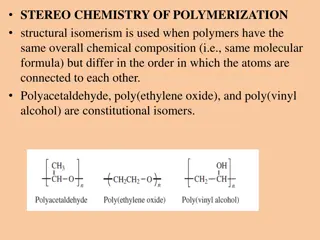
Acopolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained by copolymerization of two monomer species are sometimes called bipolymers. Those obtained from three and four monomers are called terpolymers and quaterpolymers, respectively.
Since a copolymer consists of at least two types of constituent units (also structural units), copolymers can be classified based on how these units are arranged along the chain. Linear copolymers consist of a single main chain, and include alternating copolymers, statistical copolymers and block copolymers. Branched copolymers consist of a single main chain with one or more polymeric side chains, and can be grafted, star shaped or have other architectures.
The structure of active centers in ZieglerNatta catalysts is well established only for metallocene catalysts. An idealized and simplified metallocene complex Cp2ZrCl2represents a typical precatalyst. It is unreactive toward alkenes. The dihalide reacts with MAO and is transformed into a metallocenium ion Cp2Zr+CH3, which is ion- paired to some derivative(s) of MAO. A polymer molecule grows by numerous insertion reactions of C=C bonds of 1-alkene molecules into the Zr C bond in the ion
Many thousands of alkene insertion reactions occur at each active center resulting in the formation of long polymer chains attached to the center. The Cossee Arlman mechanism describes the growth of stereospecific polymers.This mechanism states that the polymer grows through alkene coordination at a vacant site at the titanium atom, which is followed by insertion of the C=C bond into the Ti C bond at the active center.
On occasion, the polymer chain is disengaged from the active centers in the chain termination reaction. Several pathways exist for termination: Cp2+Zr (CH2 CHR)n CH3+ CH2=CHR Cp2+Zr CH2 CH2R + CH2=CR polymer Another type of chain termination reaction called - hydrogen elimination reaction also occurs periodically: Cp2+Zr (CH2 CHR)n CH3 Cp2+Zr H + CH2=CR polymer
Polymerization reactions of alkene with solid titanium-based catalysts occur at special titanium centers located on the exterior of the catalyst crystallites. Some titanium atoms in these crystallites react with organoaluminum cocatalysts with the formation of Ti C bonds. The polymerization reaction of alkenes occurs similarly to the reactions in metallocene catalysts LnTi CH2 CHR polymer + CH2=CHR LnTi CH2-CHR CH2 CHR polymer
The two chain termination reactions occurs quite rarely in Ziegler Natta catalysis and the formed polymers have a too high molecular weight to be of commercial use. To reduce the molecular weight, hydrogen is added to the polymerization reaction: LnTi CH2 CHR polymer + H2 LnTi H + CH3 CHR polymerAnother termination process involves the action of protic reagents, which can be intentionally added or adventitious.