Understanding Parts per Million (PPM) in Solutions
Explore the concept of Parts per Million (PPM) in solutions, how it differs from percentages, and practice calculations to express solubilities in PPM. Learn to calculate PPM for different scenarios involving solutes dissolved in solvents, such as gases in water. Master the formula and application of PPM for accurate concentration measurements in chemistry.
Uploaded on Oct 09, 2024 | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
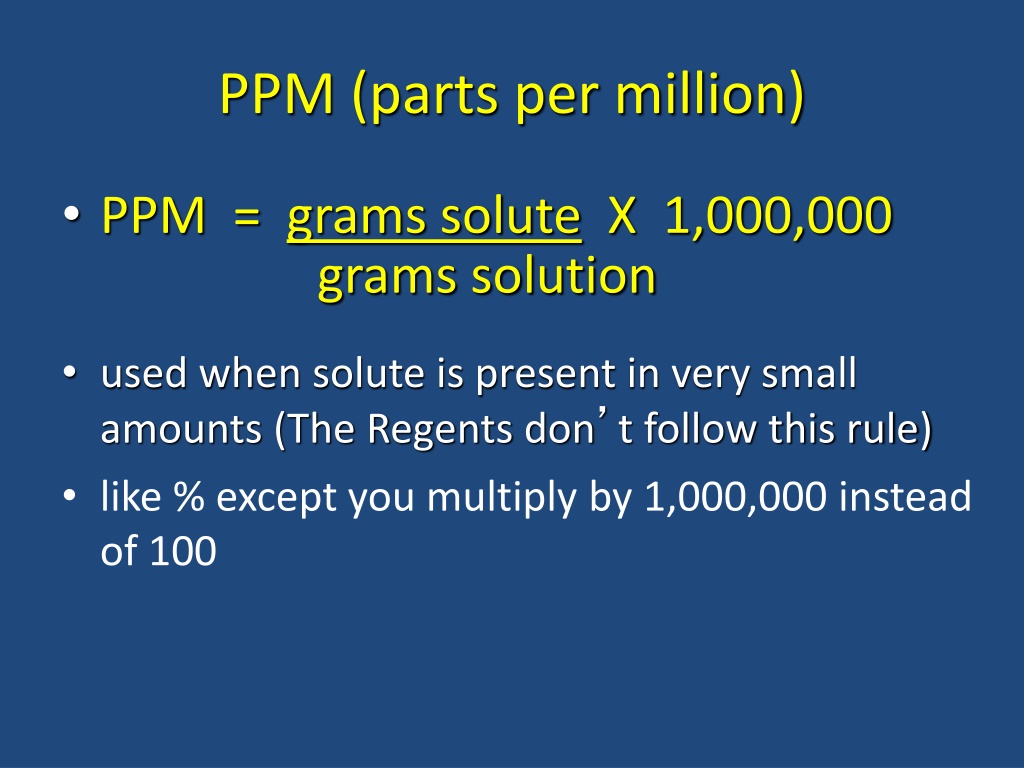
PPM (parts per million) PPM = grams solute X 1,000,000 grams solution used when solute is present in very small amounts (The Regents don t follow this rule) like % except you multiply by 1,000,000 instead of 100
LETS PRACTICE: About 0.0043 g of O2 can be dissolved in 100 mL of water at 20oC. Express in ppm. [Memorize: 1 mL water has mass of 1 g] What is the solvent? Water How much solvent is there? 100 mL = 100 g How much solute is there? 0.0043g How much is the total solution? 100 g + .0043 g = 100.0043g
LETS PRACTICE: About 0.0043 g of O2 can be dissolved in 100 mL of water at 20oC. Express in ppm. [Memorize: 1 mL water has mass of 1 g] THE EQUATION ppm = 0.0043g X 1,000,000 100.0043g ppm = 43 ppm
NOW YOU TRY: CO2 gas has solubility of 0.0972 g/100 g water at 40oC; Express in parts per million ppm = 0.0972 g solute X 1,000,000 100.0972 g solution = 971 ppm
HERES ANOTHER: What is the concentration in ppm of a solution with 30.0 g NaNO3 in 70.0 g water? ppm = 30.0 g NaNO3 X 1,000,000 100.0 g solution = 300,000 = 3.0 X 105 ppm
LAST ONE: How many grams of KOH are needed to be dissolved in water to make 2000.0 grams of a 10.0 ppm solution? 10 ppm = X grams KOH X 1,000,000 2000.0 g solution X = 0.02 grams KOH