Understanding Mixtures: Types and Examples
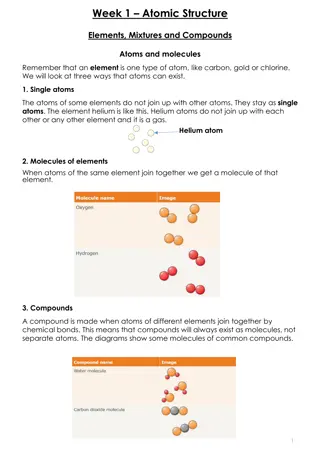
A mixture is a combination of different ingredients that can be separated. There are various types of mixtures such as liquid solutions, solid solutions, and gas solutions. Liquid solutions involve solid substances dissolved in a liquid, like sugar in water, while solid solutions include metal alloys like steel. Gas solutions like air contain a mixture of gases. Soluble substances dissolve in liquids, and their dissolution can be affected by factors like temperature and stirring. Understanding the concept of saturation point in solutions is also crucial. Explore the fascinating world of mixtures and solutions!
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
What is a mixture? A mixture is a combination of different ingredients. In order to be a mixture, the ingredients must be able to be separated again. Sometimes you can see all of the individual ingredients in a mixture, as in this bowl of sweets.
Mixtures liquid solutions Sometimes you cannot see all of the individual ingredients in a mixture. A liquid solution is a type of mixture that contains a solid substance dissolved in a liquid. For example, when sugar dissolves in water, we cannot see the sugar any more.
Mixtures solid solutions You probably know that a metal alloy is a mixture of two or more different types of metal. But did you know that a metal alloy is actually a solid solution? The alloy steel is an example of a solid solution.
Mixtures gas solutions Solutions can be made up of gases too. Air is a gas solution. It is a mixture of oxygen, carbon dioxide and other gases.
Liquid solutions and soluble substances We know that a liquid solution contains a solid substance dissolved in a liquid. A substance that dissolves in liquid is described as being soluble. Sugar is soluble. There is a lot of sugar in fizzy drinks, but you cannot see it because it has dissolved.
Liquid solutions and soluble substances Some substances dissolve faster in warm water than in cold water. You can test sugar to see if it dissolves faster in warm water than in cold water.
Liquid solutions and soluble substances It also interesting to investigate if sugar will dissolve any faster in water by stirring it. Solutions can be affected and changed by various actions.
Liquid solutions and saturation point At a certain point, a substance will stop dissolving in a liquid if there is too much of the substance. The is called the substance s saturation point. When it reaches its saturation point, you will see some undissolved substance sitting at the end of the container.
Liquids and insoluble substances When a substance does not dissolve in any liquid, it is described as being insoluble. For example, sand is an insoluble substance. Some substances are not water-soluble. For example, oil is not water-soluble. If you pour oil into water, you will see that they do not mix.
Changing substances reversible changes We can make certain changes to a substance that can be undone, or reversed. Freezing is a reversible change. You can freeze water to make ice, but you can reverse this by letting the ice melt.
Changing substances reversible changes Evaporation is another reversible change. When we boil water, it evaporates as steam. However, the steam condenses back into liquid (water) once it touches a cold surface
Changing substances irreversible changes Mixing substances can sometimes cause an irreversible change. A change is irreversible if it cannot be undone. When you mix the dry and liquid ingredients for a cake the mixture changes to batter.
Changing substances irreversible changes After you bake the cake, it is permanently changed. The baked cake is no longer a mixture because the ingredients cannot be separated.
Illustrations Shutterstock Beehive