Understanding Chemical Characteristics and Units in Water Composition Analysis
Discover the essential chemical characteristics required to plot a water sample on a diagram and learn about the limitations of using molarity for concentrated solutions of divalent ions, especially when dealing with changing temperatures. Explore the concept of molality and its advantages, while also delving into the conversion between molarity and molality for dilute solutions. Gain insights into the importance of considering ppm in water composition analysis and its equivalence to mg/L.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
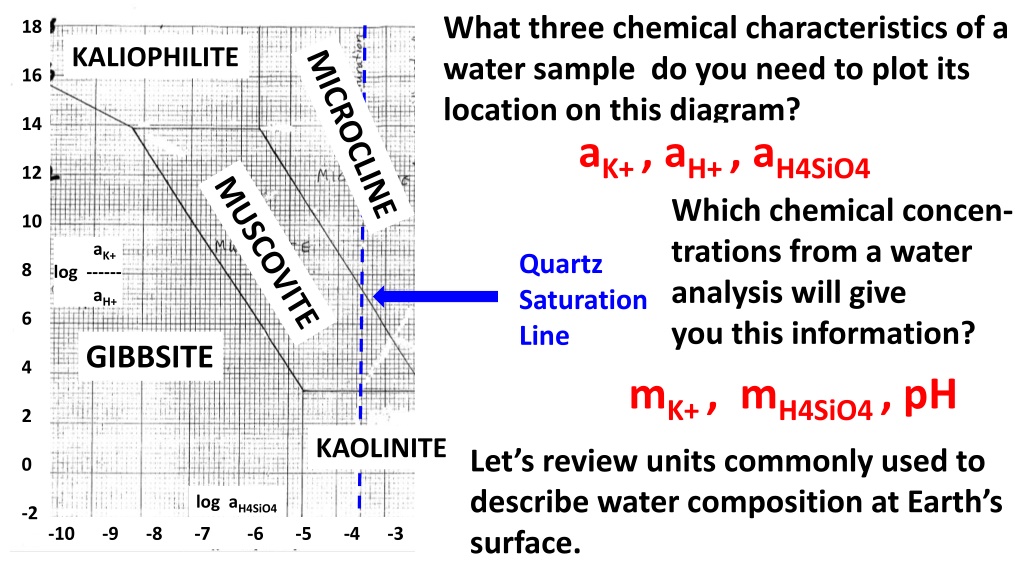
What three chemical characteristics of a water sample do you need to plot its location on this diagram? aK+ , aH+ , aH4SiO4 Which chemical concen- trations from a water analysis will give you this information? mK+ , mH4SiO4 , pH KAOLINITE 18 KALIOPHILITE 16 14 12 10 aK+ Quartz Saturation Line 8 log ------ aH+ 6 GIBBSITE 4 2 Let s review units commonly used to describe water composition at Earth s surface. 0 log aH4SiO4 -2 -10 -9 -8 -7 -6 -5 -4 -3
molarity (M) = moles solute per liter of solution Quick, easy, useful for dilute solutions, room temperatures, and solutes that don t strongly attract H2O molecules. https://chem.libretexts.org/Courses/Prince_Georges_Community_College/CHEM_2000%3A_Chemistry_for_En gineers_(Sinex)/Unit_4%3A_Nomenclature_and_Reactions/Chapter_12%3A_Aqueous_Reactions/Chapter_12.1 %3A_Preparing_Solutions
For one of my research projects I had to make artificial seawater, so I made a solution of 1M MgSO4 6H2O using the method just described. I weighed and added the solid solute and filled with distilled water to the mark on the flask as indicated here. After swirling to stir the solution level had decreased significantly. What do you think happened after adding a high concentration of a readily hydrated, divalent cation?
Water molecules were strongly drawn to the surface of vast numbers of Mg2+ ions, thereby decreasing the volume of the solvent.Clearly, making concentrated solutions of divalent ions is problematic with units of molarity. It also turns out that using molarity to make solutions at widely different temperatures yields questionable results. Why do situations involving changing temperature indicate another unit should be used?solvent volumes change with temperature
molality (m) = moles solute per kilogram of water https://slidetodoc.com/chapter-12-preview-objectives- solutions-suspensions-colloids-solutes-2/
molarity is essentially equal to molality for dilute solutions at 25oC ppm = parts per million by weight so it equals milligrams of solute in how much water? 1 kilogram At 25oC what is the volume of 1 kg of water? 1 liter So, mg/L is equivalent to ppm in dilute solutions Be careful when using ppm because it usually means parts per million by volume for gases.
Besides obtaining a water analysis reported in mg/L what other steps will you need to complete to plot a water chemistry on the diagram? Convert mg/L to molarity Calculate Ionic Strength Determine Activity Coefficient for K+ Calculate aH+ from pH How will you decide which version of the Debye-Huckel equation to use to calculate the activity coefficient? Use Ionic Strength for simplicity we will use molarity instead of molality
You will find this website very helpful for checking your work, however make sure you know how to do the calculations of Ionic Strength and activity coefficient with your calculator so you can complete problems on problem sets. https://www.lenntech.com/calculators/activity/activity-coefficient.htm
Used for I < 0.01 Azi2 I log i = - ------------------ 1 + Bai I For 25oC: A = 0.5092, B =0.3283, & aK+ = 3 0.0114 - ----------- 1.0221 0.5092(1)2 0.0005 - -------------------------------- = 1 + 0.3283(3) 0.0005 log K+= log K+= - 0.0112 K+ = 0.975
For I < 0.005 the expression reduces to log i = -Azi2 I log K+ = ?? -0.5092(1)2 0.0005 = -0.0114 K+ = ? 0.974 Calculate coordinates for the point
aK+ = [K+]K+ = ? 2.79 X 10-5 * 0.974 2.72 X 10-5 =3.16 X 10-8 10-7.5 aK+ = ? pH = 7.5, so aH+ = ? aK+ ? ---- = ------------- = ? aH+ ? aH4SiO4 = 0.00027, so,log aH4SiO4 = ? aK+ 2.72 X 10-5 log ---- = ? aH+ 2.93 860.8 3.16 X 10-8 -3.57
So, coordinates of point representing the analysis are: log aH4SiO4 = -3.57 log aK+ /aH+ = 2.93 18 KALIOPHILITE 16 14 12 10 aK+ 8 log ------ aH+ 6 GIBBSITE 4 2 Which mineral is stable? kaolinite KAOLINITE 0 log aH4SiO4 -2 -10 -9 -8 -7 -6 -5 -4 -3