Understanding Matter: Properties, Changes, and Classification
Explore the classification of properties and changes in matter, including physical and chemical properties, extensive and intensive properties, pure substances versus mixtures, and homogeneous versus heterogeneous mixtures. Learn about pollution-producing processes involving physical changes and the differences between mixtures and pure substances.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
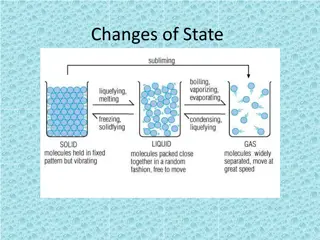
MATTER,PHASES, PHYS/CHEM CHANGES AND PROPERTIES Warm-Ups
Classify the following as either a physical or chemical property or change. Write the letter P by physical properties and C by chemical properties. Oxygen gas supports combustion, accompanied by flames Hydrogen gas has the potential to ignite and explode. Water boils below 100 C on top of a mountain. Lead is denser than aluminum. Salt dissolves in water. Zinc reacts with hydrochloric acid and produces bubbles. A plastic bottle is buoyant in water. Two solutions are mixed together and produce a precipitate. A piece of rock is crushed into smaller pieces. A leaf changes color.
Physical Properties can either be classified as extensive or intensive. Extensive properties depend on the amount of substance, where intensive properties do not depend on the amount of substance. Place an (E) by the extensive properties and an (I) by the intensive properties. Mass Color Length Density Volume Height Weight Buoyancy Viscosity Ductility
Pure Substances have a constant composition and consistent properties. Mixtures have variable composition and inconsistent properties. Pure substances tend to be separated chemically while mixtures can be separated physically. Label Pure Substances with (PS) and Mixtures with (M). H K Steel (made with Fe and C) Brass (made with Cu and Zn) NaCl H2O Concrete Iron fillings and sulfur powder Magnesium ribbon in HCl solution Chex Mix
Mixtures are either homogeneous (uniform in composition throughout) or heterogeneous (not uniform in composition throughout). Heterogeneous mixtures have visible identifiable components, where in homogeneous mixtures you cannot identify the components. Label the following mixtures with (Het) for heterogeneous or (Hom) for homogeneous. Kool-aid Gravel Ocean water Concrete Carbonated water Tap Water Distilled Water Metal Alloys such as Beryllium Copper Air in the atmosphere Water with blue food coloring mixed in
Which of these describes a pollution producing process that involves only a physical change? A.Coal with high sulfur content is burned, producing gases that cause acid rain. B.Chlorofluorocarbons are released, changing ozone in the upper atmosphere into oxygen. C.Hot wastewater is discharged into a lake, lowering oxygen levels in the water D.Nitrogen oxide emissions combine with water vapor, producing nitric acid Which of the following statements describes a difference between a mixture and a pure substance? A.A mixture needs to be homogenous, while a pure substance tends to be heterogeneous. B.A mixture has a specific melting point, while the melting point of a pure substance varies. C.The density of a mixture cannot change with temperature, but the density of a pure substance can change. D.The composition of a mixture can vary from sample to sample, but the composition of a pure substance is always the same.
Determine whether each of the following is a/an: chemical property intensive physical property extensive physical property Indicate whether each of the following is a A. pure substance B. mixture 1. Dry Ice (solid carbon dioxide) 2. Aluminum foil 1. Corrosive 3. Brass 4. Liquid Nitrogen 2. Boiling point = 78 C 5. A sample of black and silver powder melts from 917 C 2021 C and can be separated using a magnet. 3. Density = 0.66 g/cm3 4. Reacts with an acid to produce a 6. An unknown silvery powder has a single melting point and does not chemically or physically separate into other substances. gas 7. A colorless liquid that boils at 79 C and can be chemically decomposed into carbon, hydrogen, and oxygen 5. Volume = 4.2 mL 6. Magnetic