Understanding Electron Configuration and Lewis Dot Structures
Learn about electron configuration using Slater's Rules to determine effective nuclear charge (Z*), and explore Lewis dot structures for elements to understand octet rules and bonding. Dive into the steps for writing Lewis structures with examples like CO2, emphasizing electron distribution and formation of double or triple bonds as needed.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
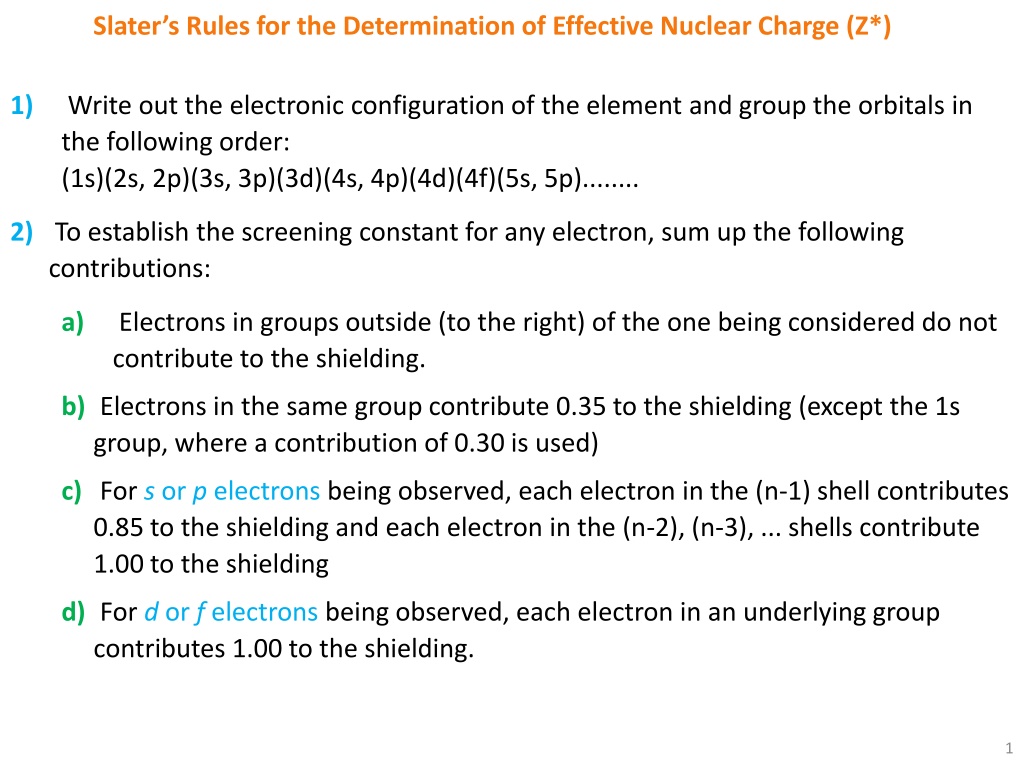
Slaters Rules for the Determination of Effective Nuclear Charge (Z*) 1) Write out the electronic configuration of the element and group the orbitals in the following order: (1s)(2s, 2p)(3s, 3p)(3d)(4s, 4p)(4d)(4f)(5s, 5p)........ 2) To establish the screening constant for any electron, sum up the following contributions: a) Electrons in groups outside (to the right) of the one being considered do not contribute to the shielding. b) Electrons in the same group contribute 0.35 to the shielding (except the 1s group, where a contribution of 0.30 is used) c) For s or p electrons being observed, each electron in the (n-1) shell contributes 0.85 to the shielding and each electron in the (n-2), (n-3), ... shells contribute 1.00 to the shielding d) For d or f electrons being observed, each electron in an underlying group contributes 1.00 to the shielding. 1
Lewis Dot Structures Each dot represents a valence electron. electron configuration of O is: Lewis symbol is: Lewis Symbol for Period 2 elements: Ne with 8 valence electrons is particularly stable because it has a full outer level, i.e. 8 dots, an octet. Helium is somewhat of an exception. It has only two dots (a duet). For He, a duet represents a stable electron configuration. Ionic Bond:If electrons are transferred, as occurs between a metal and a nonmetal Covalent Bond: If the electrons are shared, as occurs between two nonmetals 2
Lewis Dot Structures Octet Rule:In either ionic or covalent bonding, the bonding atoms obtain stable electron configurations; since the stable configuration is usually 8 electrons in the outermost shell, this is known as the octet rule. Steps for Writing Lewis Structures: 1) Write the correct skeletal structure for the molecule. H atoms are always terminal. Put the more electronegative elements in terminal positions and the less electronegative elements (other than H) in the central position. 2) Calculate the total number of electrons for the Lewis structure by summing the valence electrons of each atom in the molecule. For ions, add one electron for each negative charge and subtract one electron for each positive charge. 3
Lewis Dot Structures 3) Distribute the electrons among the atoms, giving octets (or duets in the case of hydrogen) to as many atoms as possible. Begin by placing 2 electrons between every 2 atoms. Then distribute the remaining electrons as lone pairs, first to terminal atoms, and then to the central atom, giving octets (or duets for H) to as many atoms as possible. 4) If any atoms lack an octet, form double or triple bonds as necessary to give them octets. Do this by moving lone electron pairs from terminal atoms into the bonding region with the central atom. 4
Lewis Dot Structures Writing Lewis Structure for CO2: Step 1: Because carbon is the less electronegative atom, put it in the central position. Step 2: Total number of electrons for Lewis structure = no. of valence e- for C + 2 x (no. of valence e- for O)= 4 + 2 x 6 = 16 Step 3: Bonding electrons are first. (4 of 16 electrons used) Lone pairs on terminal atoms are next. (16 of 16 electrons used) Step 4: Since carbon lacks an octet, move lone pairs from the O atoms to bonding regions to form double bonds. 5
Resonance This concept is used when two or more valid Lewis structures can be drawn for the same compound. Example: The following two Lewis structures of O3 are equally correct: Experimentally, the bonds in the O3 molecule are equivalent and each is intermediate in strength and length between a double bond and single bond. Therefore,the molecule is represented with both structures, called resonance structures, with a double-headed arrow between them: Resonance Structure: It is one of two or more Lewis structures that have the same skeletal formula, but different electron arrangements. 6
Resonance Resonance Hybrid: The actual structure of the molecule is intermediate between the two (or more) resonance structures and is called a resonance hybrid. Resonance Stabilization: In the resonance hybrid structure, the electrons are more spread out (or delocalized) than in any of the resonance structures. The resulting stabilization of the electrons (that is, the lowering of their potential energy due to delocalization) is called resonance stabilization. Formal Charge Itis a fictitious charge assigned to each atom in a Lewis structure that helps us to distinguish among competing Lewis structures. Definition: It is the charge it would have if all bonding electrons were shared equally between the bonded atoms. The formal chargesof H and F in HF (the calculated charges if we ignore their differences in electronegativity) are both zero. 7
Formal Charge Calculation of Formal Charge: It can be calculated as the difference between the number of valence electrons in the atom and the number of electrons that it owns in a Lewis structure. Formal charge = no. of valence electrons - (no. of nonbonding electrons + no. of bonding electrons) Formal charge of H in HF = 1 [0 + (2)] = 0 Formal charge of F in HF = 7 [6 + (2)] = 0 Rules for Formal Charge: 1) The sum of all formal charges in a neutral molecule must be zero. In an ion, the sum of all formal charges must equal the charge of the ion. 3) Small (or zero) formal charges on individual atoms are better than large ones. 4) Formal charges of same sign on adjacent atoms make an unfavorable structure. 5) For better stability, more negative atoms should bear negative formal charge. 8
Formal Charge Question: Assign formal charges to each atom in the resonance forms of the cyanate ion (OCN-). Which resonance form is likely to contribute most to the correct structure of OCN-? Solution: Structures A and B have the least amount of formal charge and are therefore to be preferred over structure C. Structure A is preferred to B because it has negative formal charge on the more electronegative atom. So, structure A contributes most to correct structure of OCN-. 9
Exceptions of Octet Rule 1) Odd-Electron Species:Molecules or ions with an odd number of electrons are called free radicals. Examples: If we try to write a Lewis structure for nitrogen monoxide (having 11 e-), we can t achieve octets for both atoms: NO reacts with O2 in the air to form NO2, another odd-electron molecule (17 e-). 2) Incomplete Octets:Electron deficient molecules tend to form incomplete octets. Examples: Boron forms compounds with only 6 electrons around B, rather than 8: Other molecules having incomplete octets: 10
Exceptions of Octet Rule BF3 can complete its octet via a chemical reaction with NH3: 3) Expanded Octets:Elements in the third row of the periodic table and beyond often exhibit expanded octetsof up to 12 (and occasionally 14) electrons. Examples: In AsF5 arsenic has an expanded octet of 10 electrons, and in SF6 sulfur has an expanded octet of 12 electrons. In 3rd period elements and beyond, d orbitals are energetically accessible (they are not much higher in energy than the orbitals occupied by valence electrons) and can accommodate extra electrons. Expanded octets neveroccur in 2nd period elements. 11