The Journey of Atoms: From Big Bang to Supernovas
The evolution of atoms, from the creation of quarks and electrons in the Big Bang to the fusion of elements in stars and their explosive dissemination as supernovas, highlights the remarkable process of atom formation and distribution in the universe. Witness the transformation from subatomic particles to complex elements, shaping the cosmos into a diverse array of matter.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Atoms to Dust to Planets Image: pixabay.com Image: pictorem.com Image: commons.Wikimedia.org Written by David Ilsley
The Big Bang created quarks and electrons. Two up quarks and a down quark combined to make a proton. Two down quarks and an up quark combined to make a neutron. Images: commons.wikimedia.org
Some protons and neutrons joined together to make helium nuclei. Left over neutrons decayed to protons and electrons. Left over protons were hydrogen nuclei. Image: commons.wikimedia.org
This all happened in the first few minutes. 380 000 years later, once the temperature was down to about 3000 K, electrons combined with the nuclei to make hydrogen and helium atoms. A hydrogen atom has one proton in the nucleus orbited by one electron. A helium atom has two protons and two neutrons in the nucleus orbited by two electrons. Images: commons.wikimedia.org
The resulting hydrogen and helium gas then started to condense into blobs under gravity. The blobs condensed further into stars. Image: flickr.com
Many of the stars were very large up to 300 times the mass of the Sun. These fused their hydrogen to helium very quickly, then fused the helium to bigger atoms with more protons, neutrons and electrons: carbon, oxygen, magnesium, silicon and other elements up to iron. Iron atoms have 26 protons and 30 neutrons in the nucleus and 26 electrons around it. Image: commons.wikimedia.org
The stars then exploded as supernovas, producing the atoms heavier than iron and scattering them throughout space. So then there were atoms of every kind in space.. Image: commons.wikimedia.org
These are the various atoms. The numbers are the atomic numbers, i.e. the number of protons in the nucleus and the number of electrons around the nucleus. Apart from hydrogen, all atoms have neutrons in the nucleus too a similar number to the number of protons or a few more. Image: pixabay.com
All atoms bigger than helium were produced in stars. Astronomers call the elements made of these atoms metals . So the universe contains hydrogen, helium and metals. [Note that chemists use the word metal differently. To a chemist, iron and gold are metals, but carbon and oxygen aren t. To an astronomer, oxygen is a metal.] Since the early days of the universe, many more supernovas have occurred, providing more and more metals. About 2% of matter in the universe is now metals (in the astronomical sense).
This graph shows the relative numbers of the different atoms in the universe. Note the vertical scale is logarithmic so each graduation indicates a 100-times increase. The most common elements (atoms) are (in order of abundance): H, He, O, C, Ne, N, Mg, Si, Fe. Image: commons.wikimedia.org
The electrons going around a nucleus are arranged in shells. Shell 1, closest to the nucleus and with the lowest energy, can hold 2 electrons (2 x 1 x 1). Shell 2 can hold 8 electrons (2 x 2 x 2); Shell 3 can hold 18 electrons (2 x 3 x 3); Shell 4 can hold 32 electrons (2 x 4 x 4); and so on. The diagram shows argon with 18 protons and 22 neutrons in the nucleus and 18 electrons around it. The diagram is schematic only. The electrons are not little balls, but are defined by probability distributions which give the likelihood that the electron will be at a particular place. Image: pixabay.com
Individual electrons are actually more like this: Image: flickr.com
Atoms can sometimes lose or gain one or more electrons, usually from their outermost shell. Shells tend to be stable when they have 2, 8, 18, 32 electrons. So Argon is very stable because its outer shell has 8 electrons. Image: pixabay.com
Potassium, however, has one more proton and one more electron than argon. Not being in a shell of 2, 8 or 18, the extra electron is less stable and is easily lost to make a potassium ion with 19 protons and 18 electrons and thus a positive charge. Image: commons.wikimedia.org
Chlorine, on the other hand, has one fewer protons and one fewer electrons than argon. There are 7 electrons in the outer shell. If an extra one gets in there, it becomes stable with 8 and it tends to stay that way. This makes a chlorine ion with 17 protons and 18 electrons and thus a negative charge. Image: commons.wikimedia.org
What tends to happen if a Potassium atom bumps into a chlorine atom is that the odd electron on the potassium atom moves to the chlorine atom. This produces two stable ions, each with 8 electrons in their outer shells. Opposite charges attract. As the potassium ion is positively charged and the chlorine atom is negatively charges, they tend to stick together forming a molecule of potassium chloride, KCl. This is an example of ionic bonding and is one way atoms can stick (or bond) together.
Another way atoms can bond is by covalent bonding. A water molecule consists of two hydrogen atoms and an oxygen atom, H2O. Oxygen has 6 electrons in its outer shell and hydrogen has one. If two hydrogen atoms combine with an oxygen atom, they can share some of their outer-shell electrons as shown in the diagram. Then all outer shells are stable with 2 or 8 electrons. This bonds the atoms. This method is called covalent bonding. Image: freesvg.org
So atoms can bond ionically by donating electrons, or covalently by sharing electrons.
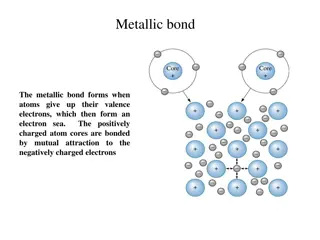
There is one other method of bonding atoms that occurs in metals like iron. When iron atoms get together to form a piece of iron metal, the atoms all lose several of their electrons and these electrons move around amongst the iron ions. The positive charge of the electrons and the negative charge of the ions hold the atoms in the metal together. This is called metallic bonding and is the common method for making pieces of metal, whether iron, copper, lead, gold or whatever. It is the free electrons which are capable of moving around that make metals good conductors of electricity and heat. Image: commons.wikimedia.org
Atoms drifting around in space behave in different ways when they bump into one another. We will consider the most common elements: H, He, O, C, Ne, N, Mg, Si, Fe. Image: commons.wikimedia.org
Helium has 2 electrons in its outer shell and neon has 8. They are very happy that way, so they don t react with anything, but just stay as single atoms. Thus He and Ne tend to stay as gas. Oxygen reacts with magnesium and silicon to produce olivine (Mg2SiO4) and with hydrogen to produce water ice (H2O). These start as tiny crystals of just a few atoms, but then further atoms hit them and they grow to form dust, maybe a small fraction of a millimetre in size. Carbon and nitrogen react with hydrogen to form methane (CH4) and ammonia (NH3). These can be ice crystals or gas molecules, depending on the temperature. Left over hydrogen atoms combine in pairs to make H2 molecules. Iron atoms tend to form small crystals of iron metal.
So we get gas consisting of H2, He and Ne, and possibly CH4 and NH3. And we get dust-sized crystals of water ice (H2O), methane (MH4), ammonia (NH3), olivine (Mg2SiO4) and iron (Fe). This gas and dust is spread throughout galaxies and, to a much lesser extent, through the space between galaxies. As galaxies rotate, though, sometimes clouds of gas and dust are concentrated by gravity. This happens particularly in the spiral arms of the Milky Way. Image: flickr.com
Here are some nebulas, concentrations of gas and dust within the Milky Way. Image: flickr.com Image: picryl.com Image: flickr.com Image: pictorem.com
You can see clouds of dust when you look at the Way in the sky. The dark patches aren t because there are no stars there; they are because the stars are obscured by dust clouds. Image: rawpixel.com
Gravity sometimes pulls parts of the gas and dust clouds together to form denser regions called bok globules. Image: flickr.com
Inside bok globules, gravity pulls gas and dust together even more to form spherical balls. The gravitational energy lost as the material in these falls together is turned into heat. The heat and pressure at the centres of these can get high enough for nuclear fusion to occur: four hydrogen nuclei (protons) join to form a helium nucleus. This releases yet more energy which heats the gas and dust up until it shines brightly. It is then a star. Image: commons.wikimedia.org
The clouds of gas and dust from which stars form are generally not motionless. For one thing they will be orbiting the galaxy. But apart from that, they are generally swirling around with some sort of average movement around the point where the star is forming. A ballerina or ice skater spins faster when she pulls her arms in closer to her axis of rotation. This conserves her angular momentum. In the same way, the matter falling in towards a star spins faster. Image: commons.wikimedia.org
At first, the gas and dust is spinning around the star in all directions. But the gas atoms and molecules and the dust particles keep bumping into one another. The result is that, eventually, they are all spinning around the same axis in the direction that the majority were moving then the star started to collapse. This produces a nearly spherical star with a flat accretion disk around it. Image: commons.wikimedia.org
Dust in the accretion disc tends to stick together. At first, electrostatic force hold the particles together. This is because the number of protons and electrons on a particle are rarely exactly balanced and so positively charged grains attract negatively charged ones. The grains form larger and larger clusters. Once they are a few metres or ten of metres across, gravitational attraction starts to play a part too and clusters group together to form bigger clusters. Once the clusters are a few kilometres across, gravity becomes the most important force. Eventually, bodies the size of terrestrial planets are formed. Image: picryl.com
High-speed impacts between protoplanets will sometimes break them up into smaller pieces, but lower-speed collisions are more likely to lead to them joining together to make a larger body. Overall, the protoplanets grow and suck up the dust and smaller bodies around them, and the accretion disk is gradually cleared of small particles. Image: commons.wikimedia.org
These planets now have sufficient gravity to attract the gases in the accretion disk. Close to the star, the gases are hot and the molecules move fast, so they tend to escape the gravity of the planets fairly easily. Further way, they are colder and move more slowly and so tend to be caught. Also, the stellar wind (the stream of radiation, protons and electrons shooting out from the star) tends to push the gases away from the inner planets towards the outer planets. The result is that the inner planets remain terrestrial, consisting of dust, which has now turned to rock and iron, and the outer planets accumulate gases (mainly hydrogen, helium, neon, water, methane and ammonia) and become gas giants. Image: flickr.com
And so, the journey from atoms to dust to planets is complete. Image: flickr.com