Pure Substances vs. Mixtures: Characteristics and Differences
Pure substances have a fixed composition and distinct properties, while mixtures vary in composition and properties. Pure substances cannot be separated into simpler substances by physical methods, whereas mixtures can be. This article explores the characteristics, distinctions, and examples of pure substances and mixtures, along with how to distinguish between a pure substance and a homogeneous mixture.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
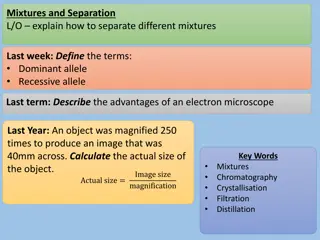
Differences between pure substances and mixtures
Characteristics of Characteristics of Pure Substances Pure Substances Fixed composition Distinct properties Cannot be separated into simpler substances by physical methods Can only be changed in identity and properties by chemical methods (Unit 4) Properties do not vary one sample to another sample
Mixtures Mixtures Form when elements and/or compounds are combined physically Properties of a mixture are related to its components Composition varies from sample to sample Can be separated by physical methods Examples of Mixtures: Tea, Perfume, Air, Salad, Beach sand, oil and vinegar salad dressing, etc.
How can we distinguish between a pure substance and an homogeneus mixture?
How can we distinguish between a pure substance and an homogeneus mixture? If pure water is heated until it boils, we will observe that temperature remains constant at 100 C during the change of state. However when water is not pure the temperature will increase progressively.
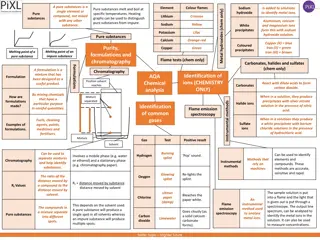
What are differences and similarities between a pure What are differences and similarities between a pure substance and a homogeneous mixture? substance and a homogeneous mixture? Pure substances Homogeneous Mixtures Consists of a single type of substance It is a system that consists of two or more pure substances Cannot substances by physical methods. be separated into other Can be separated into its components by physical methods (distillation, evaporation,chromatography..) Has its own fixed specific properties (density, boiling point, ) Mixtures shows the properties of its components and these properties change with the proportion of the components (concentration) Temperature is constant when boiling. Temperature changes when boiling,the boiling point is unclear. Examples: iron, copper, water, sodium chlorine,copper sulfate, Examples:sea water,air, sugar in water BOTH ARE HOMOGENEOUS
What would you do? Learning more Do you drink chemically pure H2O? What is the difference between chemically pure versus Clean water?
Watching videos UNICEF Tap project How far would you walk to find clean drinking water?
Working in groups What's the difference between salt and fresh water? What would you do to differentiate them?