Oil-Water Interaction and Oil Absorbing Polymers
Explore the intricacies of why oil and water don't mix, the challenges faced in containing oil spills, and the innovative solution of oil-absorbing polymers like Enviro-Bond 403. Discover the science behind non-polar substances, hydrophobicity, and the role of polymers in environmental protection.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Ryan Kiddey RET Program 2018 University of Akron OIL ABSORBING POLYMER
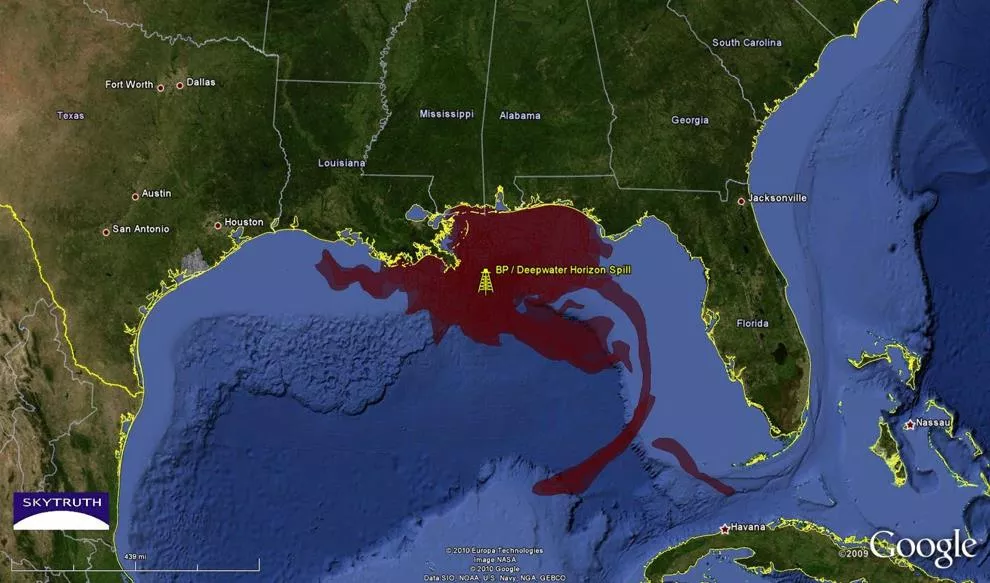
2010 BP Oil Spill Disaster Video Summary of Disaster Video of Wildlife Affected by the Disaster
What could have been done to contain the oil slick on the water?
Why doesnt Oil and Water Mix? Oil is a nonpolar substance There are many hydrogen atoms on the molecule, however they are connect to carbon (not O, N, or F)! Oil is not very attractive to strongly attractive molecules Water is a very polar substance The hydrogens are connected to oxygen which allows the molecule to potentially interact with neighboring molecules by hydrogen bonding to it. Water is very attracted to other extremely attractive molecules.
Why doesnt oil and water mix? Water molecules are STRONGLY attracted to each other due to their ability to hydrogen bond (IMF). The oil molecules will come together through very weak dispersion forces. If Oil has a slight temporary dipole (dispersion force) why won t it mix with the highly polar water??? THINK OF WATER MOLECULES AS VERY STRONG MAGNETS AND OIL MOLECULES AS VERY WEAK MAGNETS. Oil molecules have to weak of a pull on water so something stronger (like another water molecule) will partner up with it. As soon as one water molecule forms a hydrogen bond (IMF) with another water molecule the charges are canceled out and the oil isn t able to interact with the water.
Oil Absorbing Polymer Enviro-Bond 403 is a polymer that is very non-polar. This causes it to be hydrophobic (just like oil). What is a polymer? a substance that has a molecular structure consisting chiefly or entirely of a large number of similar units bonded together, e.g., many synthetic organic materials used as plastics and resins. Enviro-Bond is patented so we don t know exactly what it looks like (trade secret) however it s probably similar to the structure to the right
References http://www.noaa.gov/resource-collections/gulf-oil-spill http://www.startribune.com/us-states-finalize-settlement-with-bp-over-gulf-oil- spill/330676651/ https://www.elastec.com/products/floating-boom-barriers/fire-resistant-oil-boom/hydro- fire-boom/ http://www.wikiwand.com/en/Petroleum https://fineartamerica.com/featured/3-water-molecule-laguna-design.html https://www.quora.com/What-is-polymer-How-many-types-of-polymers-are-classified- Explain-each-polymer-with-properties-and-applications https://sl.wikipedia.org/wiki/Slika:Isotactic-polypropylene-3D-balls.png

 undefined
undefined