Office of Sponsored Projects Initiatives at University of Utah
The Office of Sponsored Projects at the University of Utah, led by Kevin Peterson, plays a key role in supporting clinical research endeavors through expert management of sponsored funding, compliance with policies and regulations, and efficient administration. The team aims to enhance research growth, maintain strong relationships, and ensure integrity and innovation in all activities.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
COMPLIANCE CAFE OFFICE OF SPONSORED PROJECTS INITIATIVES TO SUPPORT CLINICAL RESEARCH AT THE UNIVERSITY OF UTAH Kevin Peterson, JD, CRA Associate Director, Clinical Research Office of Sponsored Projects
Vision Statement The Office of Sponsored Projects provides expertise to support research growth and stewardship of sponsored funding. We foster strong and collaborative relationships through integrity, outstanding service, and communication. We increase efficiency and support compliance in research administration through innovation and technology.
Purpose Statement OSP supports faculty through effective management of extramural sponsored proposals and awards funded by federal and state agencies, foundations, and other public and private sources. OSP is primarily responsible for interpreting and ensuring compliance with University policy, proposal and award terms and conditions, and applicable federal and state laws and regulations. OSP reviews and submits proposals on behalf of the University. We also draft, negotiate, and sign sponsored agreements and subawards.
OSP Clinical Trials Team Initiatives Solidify Team Structure Expand Resources Increase Transparency Assess Performance Visible Presence
OSP Clinical Trials Team Initiatives Solidify Team Structure
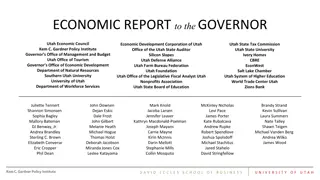
OSP ORGANIZATIONAL CHART - Historic Director Brent Brown Grants Team Assistant Ylia Lopez Industry Team Associate Director: Todd Nilsen Associate Director: Grants Kristie Thompson SPO - Grant Officers Contract Officers Timothy Tripp Project Managers TBD Todd Bjorklund (Pre-Award 25%) Erick Batres Annie Horne Elisabeth Zuniga Douglas Wawrzynski Rohmee Gonzales Jenny Hoskins Laurel Duncan (Communications 25%) Trevor Gordon Rob Potts Todd Bjorklund (75%) Isabella Johnson Chris Hardy Michael Rasmussen Katelyn Dalley (75%) Isabella Johnson (Post-Award 25%) Tara Merrill Laurel Duncan (75%) Shauna Petersen Julian Tippetts Trevor Gordon Hailey Palmer Erica Trejo (Employee Achievement 25%) Gary Christensen Mary Mulvey Kathy Christiansen Rebecca G. Contract Administrator Lona Gordon Graphics and Media Specialist Tara Mleynek Grant/Contract Administrators Steven Balls (Hailey Palmer- Replacement TBD) Staff Specialist (Legal Interns) Colton C., Hayley M., Amanda S. Computer Specialist Darren Gonzol Administrative Assistant Britney Fuger
OSP Organizational Chart: Clinical Trials Team Kevin Peterson Associate Director Trevor Gordon Sponsored Programs Officer Mike Timothy Tripp Sponsored Programs Officer Julian Tippets Sponsored Programs Officer Eric Elisabeth Zuniga Sponsored Programs Officer James Borshell Sponsored Programs Officer Jon Cantu Sponsored Programs Officer (CDAs) Tara Merrill Sponsored Programs Officer Rasmussen Sponsored Programs Officer Lazenby Sponsored Programs Officer Open
OSP Organizational Chart: Clinical Trials Team Kevin Peterson Associate Director Manager Trevor Gordon Sponsored Programs Officer Mike Timothy Tripp Sponsored Programs Officer Julian Tippets Sponsored Programs Officer Elisabeth Zuniga Sponsored Programs Officer James Borshell Sponsored Programs Officer Jon Cantu Sponsored Programs Officer (CDAs) Eric Lazenby Sponsored Programs Officer Tara Merrill Sponsored Programs Officer Rasmussen Sponsored Programs Officer Open
OSP Clinical Trials Team Initiatives Solidify Team Structure Expand Resources
Expand Resources Accelerated Clinical Trial Agreement Master Agreements Agreement templates Training materials OSP website Process clarification Clinical Trial Guidance Document
Clinical Trial Agreement Guidance Document Agreement Agreement Intake Intake Initial Initial Review Review Continued Continued Review Review Preaward Preaward Spending Spending Agreement Agreement Execution Execution
Agreement Intake
Initial Review
Continued Review
Preaward Spending
Agreement Execution
Clinical Trial Agreement Guidance Document Faculty, staff, and sponsors should contact the OSP Sponsored Projects Officer responsible for negotiating the CTA for questions or concerns about the process or timelines. Issues should be escalated to the OSP Associate Director for Clinical Research, or the OSP Director. Agreement Agreement Intake Intake Initial Initial Review Review Continued Continued Review Review Preaward Preaward Spending Spending Agreement Agreement Execution Execution
OSP Clinical Trials Team Initiatives Solidify Team Structure Expand Resources Increase Transparency
Increase Transparency Use information systems to track and manage sponsored agreements through the project lifecycle and regularly collect information and metrics related to contracting and sponsored program management.
OSP Clinical Trials Team Initiatives Solidify Team Structure Expand Resources Increase Transparency Assess Performance
Assess Team Performance Workload management and utilization of metrics to assess turnaround times and break points
OSP Clinical Trials Team Initiatives Solidify Team Structure Expand Resources Increase Transparency Assess Performance Visible Presence
Visible Presence Monthly/Quarterly meetings with individual departments to discuss negotiation updates/issues One click away: Effective use of Zoom and Microsoft Teams Participation in Research Education courses
OSP Clinical Trials Team Initiatives Solidify Team Structure Expand Resources Increase Transparency Assess Performance Visible Presence
Questions Kevin Peterson, JD, CRA Associate Director, Clinical Research Office of Sponsored Projects kevin.peterson@osp.utah.edu