Identification and Characterization of SVA Retroelement Insertions in Hereditary Cancer Panels
SVA retroelement insertions, specifically SINE-VNTR-ALU (SVA) types, were examined through Next Generation Sequencing (NGS) and RNA analysis in hereditary cancer panel testing. Twelve unique SVA retroelements were identified in genes like APC, BRCA1, CHEK2, and others, with variant interpretations ranging from pathogenic to benign. The study provides insights into the potential impact of SVA retroelements on cancer predisposition genes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Identification and characterization of SVA retroelement insertions through NGS hereditary cancer panel testing and RNA analysis. Maria Elias, Paola Nix, Shujuan Pan, Debora Mancini-DiNardo, Bradford Coffee, and Benjamin Roa Myriad Genetics, Inc., Salt Lake City, UT Disclosures: All authors are employees of Myriad Genetics, Inc., and receive salaries and stock options as compensation
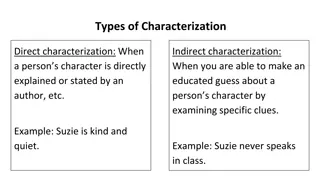
Background and Methods SINE-VNTR-ALU (SVA) insertions are the least abundant type of nonautonomous retroelement, with approximately 2700 copies in the human genome. Although challenging to identify, Next Generation Sequencing (NGS) and RNA analysis have improved their identification and characterization. While the role of SVA retroelements has been well documented in disease, there are few accounts of SVA retroelements disrupting cancer predisposition genes in germline testing. TSD (CCCTCT)n Alu-like VNTR SINE-R Poly(A) TSD Figure 1: The structure of a full length SVA retroelement includes a simple repeat sequence, an Alu-like region, a variable number of tandem repeats (VNTR), SINE-R sequence, and a Poly(A) tail. The SVA retroelement is flanked by a target site duplication (TSD) of surrounding genomic sequence. Genetic testing was performed using genomic DNA on an NGS hereditary cancer panel for sequencing and large rearrangement analysis. Blood samples for RNA analysis were obtained according to our laboratory s IRB-approved research protocol. Kazazian and Moran, 2017 Hancks and Kazazian, 2016
Twelve unique SVA retroelements identified in hereditary cancer panel testing Gene APC APC BRCA1 CHEK2 PMS2 MLH1 ATM CHEK2 PMS2 PMS2 PMS2 BMPR1A Variant Interpretation Pathogenic Pathogenic Pathogenic Pathogenic Pathogenic Pathogenic Uncertain Uncertain Uncertain Uncertain Uncertain Benign Finds 1 5 1 4 >50 7 1 1 2 1 1 >100 c.118_119insSVA c.1958+41_1958+42insSVA c.3094_3095insSVA c.966_967insSVA c.804-60_804-59insSVA* c.760_761insSVA c.8010+15_8010+16insSVA c.593-3_593-2insSVA c.804-60_804-59insSVA_0.5kb c.804-61_804-60insSVA (full) c.804-61_804-60insSVA (truncated) c.230+11_230+12insSVA Table 1: SVA retroelement insertions identified in hereditary cancer panel testing. * van der Klift et al., 2012
APC c.1958+41_1958+42insSVA a pathogenic variant associated with FAP Alt donor ~SVA~ Genomic DNA Exon 14 Exon 15 Int14-truncated SVA cDNA Exon 14 Exon 15 GCTACAAATGAGGACCACAGgtatatatagagttttatattacttttaaagtacagaattcGGCGACAGAGCGAGACTCCATCTCAAAAAAAA ORF: GCT ACA AAT GAG GAC CAC AG//G TAT ATA TAG Figure 2: Proposed mechanism of pathogenesis for APC c.1958+41_1958+42insSVA.
BMPR1A c.230+11_230+12insSVA a benign variant that does not affect RNA splicing ~SVA~ Genomic DNA Exon 2 Exon 3 CACATGCATgtaagtattttTTTTTTTTTTTTTTT .CGCGGCGGCAAAGACTGCATgtaagtattttatgc SVA TSD TSD (duplicated splice donor) (endogenous splice donor) cDNA Exon 2 Exon 3 CACATGCAT|AACTAATGG Figure 3: Proposed mechanism resulting in a benign interpretation for BMPR1A c.230+11_230+12insSVA .
Conclusions SVA retroelement insertions are technically challenging variants whose identification can be enhanced by comprehensive NGS analysis in hereditary cancer panels. Follow-up RNA analysis demonstrates the variable effects of these variants, that can result in a pathogenic or benign interpretation depending on their location and sequence context. These findings highlight a previously underappreciated mechanism of pathogenesis in cancer predisposition genes, expanding their mutational spectrum and leading to improved clinical management for patients and their family members.