Exploring the States of Matter Through Kinetic Molecular Theory
Delve into the fascinating world of matter states with the Kinetic Molecular Theory. Learn about the properties and behaviors of solids, liquids, gases, and plasma. Understand how particle movement, energy, and spacing differ across these states, shaping their unique characteristics.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Ch. 3 - Matter States of Matter Kinetic Molecular Theory States of Matter
Lesson Objectives I can describe the 4 states of matter in terms of KMT (the energy of the particles and the force of attraction between those particles. I can diagram the way particles will look inside a solid, liquid, and gas.
Kinetic Molecular Theory KMT Particles of matter are always in motion. The kinetic energy (speed) of these particles increases as temperature increases.
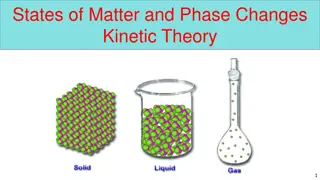
4 States of Matter Solids Liquids Gases Plasma
Four States of Matter: Solids Solids very low KE particles vibrate but can t move around Definite shape Definite volume Can t be compressed
Four States of Matter: Liquids Liquids Medium KE particles slide around but are still close together Indefinite Shape Definite Volume Can t be Compressed
Four States of Matter: Gases Gases high KE - particles can separate and bounce throughout container Indefinite shape Indefinite volume Can be Compressed
Four States of Matter: Plasma Plasma very high KE - particles collide with enough energy to break into charged particles (+/-) gas-like, indefinite shape & volume Magnetic fields affect them stars, fluorescent light bulbs, CRTs
Chart of 4 States of Matter State of Matter Shape Volume Movement Compressed? Energy Particle Spacing Solid Liquid Gas Plasma Definite Definite Vibrate No Low Indefinite Definite Slide No Middle Indefinite Indefinite Bounce Yes High Indefinite Indefinite Bounce Yes Very High Tight Loose Medium particles close but move Spread out Spread out Attraction Strong Hardly any Hardly any
Big Ideas The state of matter is determined by how fast the particles are moving and the attraction between them. Kinetic Molecular Theory All substances are in constant motion and the amount of motion is proportional to the temperature.