Energy - Forms, Calculations, and Applications
Explore the concept of energy through various images, including forms of energy, kinetic versus potential energy, and calculations involving kinetic and potential energy. Learn about identifying energy states, calculating kinetic energy, and solving physics problems related to energy transfer. Dive into examples like determining speed from energy and calculating potential energy from height. Discover how energy plays a crucial role in everyday scenarios, such as roller coasters and falling objects.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
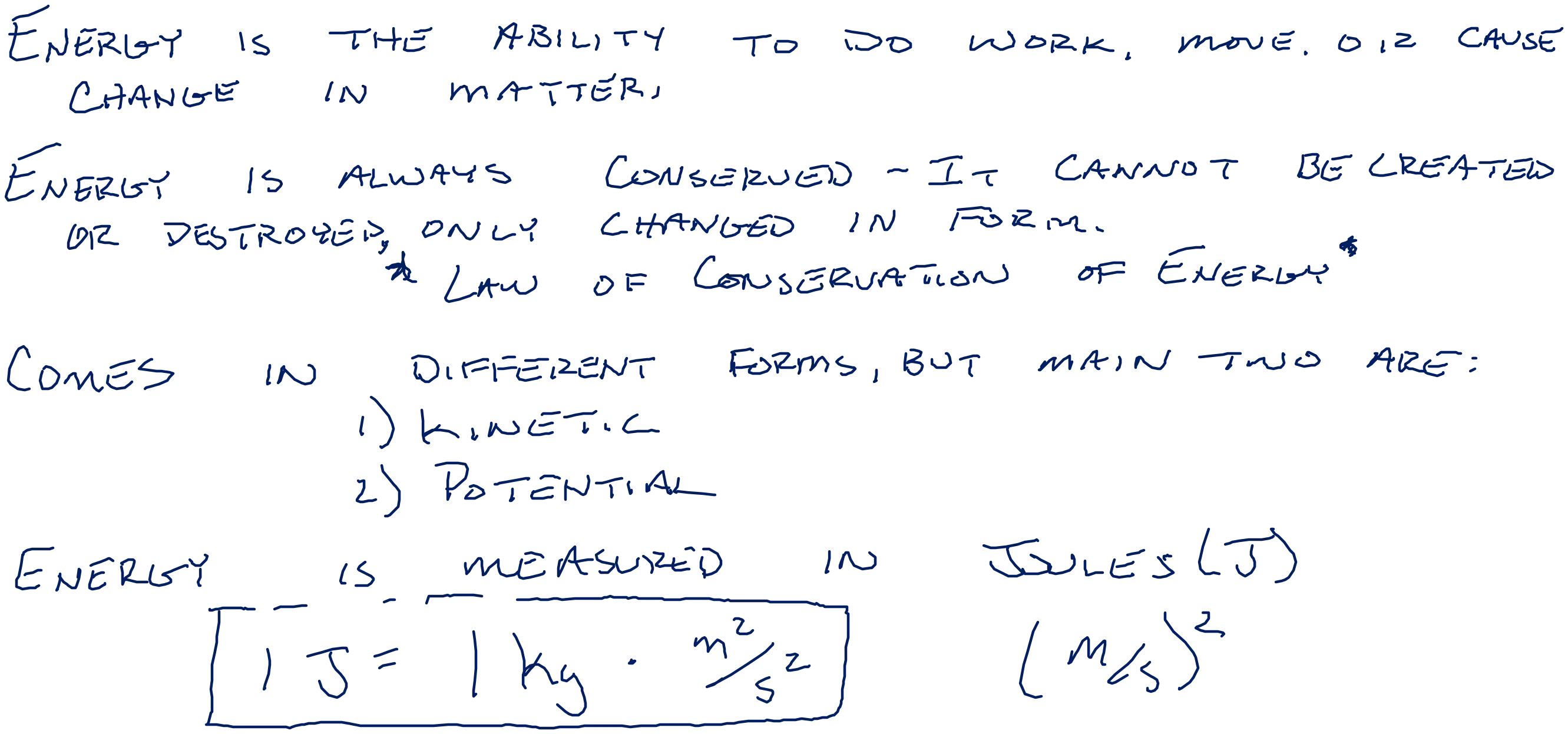
1. Which letter shows the ball when it has the maximum kinetic NRG ? 2. Which letter shows the ball when it has the maximum potential NRG ? 3. Which letter shows the ball when it has the least potential NRG? 4. Which letter shows the ball when it has the least kinetic NRG? 5. Which letter shows the ball when it has just a little more kinetic NRG than A? 6. Which letter shows the ball when it has just a little more potential NRG than letter C? 7. Which letter shows the ball when it has just a little less potential energy than letter F? 8. Which letter shows the ball when it has just a little more kinetic energy than letter G? 9. Which letter shows the ball when it has just a little less kinetic energy than letter D? 10. Which letter shows the ball when it has just a little less potential energy than letter C? 11. Which sequence correctly shows an increase in potential energy? 13. Which sequence correctly shows an decrease in kinetic energy? A. E, F, B, G C. D, E, B, F B. B, F, E, C D. A, G, F, C A. E, F, B, G B. B, F, E, C C. D, E, B, F D. A, G, F, C 14. Which sequence correctly shows an decrease in potential energy? A. E, F, B, G C. D, E, B, F 12. Which sequence correctly shows an increase in kinetic energy? B. B, F, E, C D. A, G, F, C A. E, F, B, G B. B, F, E, C C. D, E, B, F D. A, G, F, C
Calculations 1. Determine the kinetic energy of a 1000-kg roller coaster car that is moving with a speed of 20.0 m/s. 2. If the roller coaster car in the above problem were moving with twice the speed, then what would be its new kinetic energy?
Calculations 15,000 J just prior to hitting the bucket of water. If Missy's mass is 50 kg, then what is her speed? A cart is loaded with a brick and pulled at constant speed along an inclined plane to the height of a seat-top. If the mass of the loaded cart is 3.0 kg and the height of the seat top is 0.45 meters, then what is the potential energy of the loaded cart at the height of the seat-top?
A lump of ice falls from an aeroplane as it comes in to land. If the ice hits the ground with a vertical speed of 85m/s, what was the height of the plane when the ice fell off? (Assume that friction can be ignored.)
Physics student is dropped (dont ask why or you re next). If they reach the floor at a speed of 3.2 m/s, from what height did they fall?
A heavy object is dropped from a vertical height of 8.0 m. What is its speed when it hits the ground?
A bowling ball is dropped from the top of a building. If it hits the ground with a speed of 37.0 m/s, how tall was the building?
A safe is hurled down from the top of a 1.3 x 102 m building at a speed of 11.0 m/s. What is its velocity as it hits the ground?
5.0 g of copper was heated from 20C to 80C. How much energy was used to heat Cu? (Specific heat capacity of Cu is 0.092 J/g C)
How much heat is absorbed by 20g granite boulder as energy from the sun causes its temperature to change from 10 C to 29 C? (Specific heat capacity of granite is 0.1 J/g C)
How much heat is released when 30 g of water at 96 C cools to 25 C? The specific heat of water is 1 J/g C.
If a 3.1g ring is heated using 10.0 calories, its temperature rises 17.9 C. Calculate the specific heat capacity of the ring.
The temperature of a sample of water increases from 20 C to 46.6 C as it absorbs 5650 calories of heat. What is the mass of the sample? (Specific heat of water is 1.0 cal/g C)
A 4.50 g coin of copper absorbed 54 calories of heat. What was the final temperature of the copper if the initial temperature was 25 C? The specific heat of copper is 0.092 cal/g C.
A 245.7g sample of metal at 75.2 degrees Celsius was placed in 115.43g water at 22.6 degrees Celsius. The final temperature of the water and metal was 34.6 Celsius. If no heat was lost to the surroundings what is the specific heat of the metal?
Determine the final temperature when a 25.0g piece of iron at 85.0 C is placed into 75.0grams of water at 20.0 C. The specific heat of iron is 0.450 J/g C. The specific heat of water is 4.18 J/g C.
Example Quiz 1. Calculate the kinetic energy of a 5 kg object moving with a velocity of 10 m/s. Calculate the potential energy of an object that weighs 12 kg and sits 15m high. Calculate the velocity of an object with 4,000J and a mass of 16 kg. Calculate the height of an object with a mass of 20 kg and 1,500J. Calculate the quantity of heat for 150g of water that increases temperature from 4oC to 40oC. Calculate the final temperature of a 5kg piece of iron (.444 J/goC) with 650J of energy if the initial temperature is 21oC. A 212.2 g sample of metal at 68oC Celsius was placed in 79.5g water at 14.2oC. The final temperature of the water and metal was 34.6 Celsius. If no heat was lost to the surroundings what is the specific heat of the metal? Calculate the velocity of an object falling from a height of 1,200 m if the object s mass was 15kg. 2. 3. 4. At which number is KE the greatest? 5. At which number is PE the greatest? 6. At which number would PE = KE? 7. 8.
Roller Coaster Lab 1) Challenge to design a roller coaster that a marble is able to pass through. The track must have at least one loop and two hills. 2) On white copy paper, draw a picture of the coaster. Be sure you label the transformation of KE to PE and vice versa.