Efficacy of Glecaprevir and Pibrentasvir in Cirrhosis
Study on the efficacy of glecaprevir and pibrentasvir in genotypes 1 and 3 cirrhosis patients, with promising SVR12 rates. Baseline characteristics, relapse data, and resistance analysis provided. The treatment showed high efficacy even in patients with baseline substitutions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
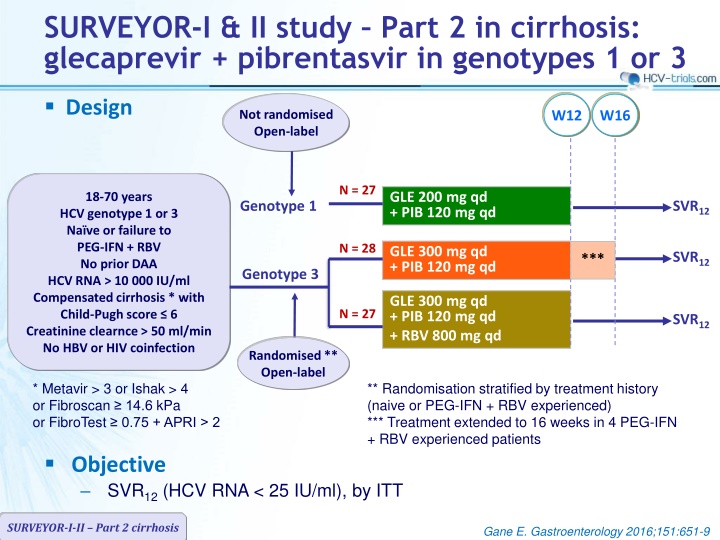
SURVEYOR-I & II study Part 2 in cirrhosis: glecaprevir + pibrentasvir in genotypes 1 or 3 Design Not randomised Open-label W12 W16 N = 27 GLE 200 mg qd + PIB 120 mg qd 18-70 years HCV genotype 1 or 3 Na ve or failure to PEG-IFN + RBV No prior DAA HCV RNA > 10 000 IU/ml Compensated cirrhosis * with Child-Pugh score 6 Creatinine clearnce > 50 ml/min No HBV or HIV coinfection Genotype 1 SVR12 N = 28 GLE 300 mg qd + PIB 120 mg qd SVR12 *** Genotype 3 GLE 300 mg qd + PIB 120 mg qd + RBV 800 mg qd N = 27 SVR12 Randomised** Open-label * Metavir > 3 or Ishak > 4 or Fibroscan 14.6 kPa or FibroTest 0.75 + APRI > 2 ** Randomisation stratified by treatment history (naive or PEG-IFN + RBV experienced) *** Treatment extended to 16 weeks in 4 PEG-IFN + RBV experienced patients Objective SVR12(HCV RNA < 25 IU/ml), by ITT SURVEYOR-I-II Part 2 cirrhosis Gane E. Gastroenterology 2016;151:651-9
SURVEYOR-I & II study Part 2 in cirrhosis: glecaprevir + pibrentasvir in genotypes 1 or 3 Baseline characteristics Genotype 3 GLE 300 + PIB 200 + RBV 800 N = 27 Genotype 1 GLE 200 + PIB 120 N = 27 Genotype 3 GLE 300 + PIB 200 N = 28 Mean age, years 58.9 55.2 55.7 Female, % 26 46 33 Race, white, % 89 93 89 Mean BMI, kg/m2 27.7 27.8 27.0 Mean HCV RNA, log10IU/ml 6.6 6.4 6.2 Genotype 1a / 1b / undetermined, % 74 / 26 / 0 - - Genotype 3a / 3b / undetermined, % - 86 / 4 / 11 100 / 0 / 0 IL28B CC, % 15 43 37 Treatment-na ve, % 78 86 89 NS3 substitutions at baseline, % 41 24 NS5A substitutions at baseline, % 19 22 SURVEYOR-I-II Part 2 cirrhosis Gane E. Gastroenterology 2016;151:651-9
SURVEYOR-I & II study Part 2 in cirrhosis: glecaprevir + pibrentasvir in genotypes 1 or 3 SVR12(HCV RNA < 25 IU/ml), % (95% CI) 100 96 96 % (88-100) (82-99) (82-99) 100 Genotype 1, 12 weeks Genotype 3, 12 weeks Genotype 3, 12 weeks 75 50 25 27 28 27 N= 0 GLE 200 + PIB 120 GLE 300 + PIB 120 GLE 300 + PIB 120 + RBV SURVEYOR-I-II Part 2 cirrhosis Gane E. Gastroenterology 2016;151:651-9
SURVEYOR-I & II study Part 2 in cirrhosis: glecaprevir + pibrentasvir in genotypes 1 or 3 Relapses NS3 RASs NS5A RASs Time of relapse Genotype Naive Gender Baseline Failure Baseline Failure Post- treatment W4 L31M + Y93N (195-fold EC50 to PIB) 1a Yes Female I170V I170V L31M Post- treatment W2 M28G (replicon not viable in vitro) 3 No Male A166S None None Resistance analysis (population sequencing with 15% threshold) SVR12was achieved in 100% of patients without baseline substitutions and efficacy remained high in the presence of baseline substitutions All 11 genotype 3-infected patients with baseline NS5A substitutions, including 4 patients with additional NS3 substitutions, achieved SVR12 SURVEYOR-I-II Part 2 cirrhosis Gane E. Gastroenterology 2016;151:651-9
SURVEYOR-I & II study Part 2 in cirrhosis: glecaprevir + pibrentasvir in genotypes 1 or 3 Adverse events and laboratory abnormalities, % Genotype 3 GLE 300 + PIB 120 + RBV 800 N = 27 Genotype 1 GLE 200 + PIB 120 N = 27 Genotype 3 GLE 300 + PIB 120 N = 28 Any adverse event 52 86 85 Serious adverse event 4 (N = 1) 7 (N = 2) 7 (N = 2) Adverse event leading to study discontinuation 0 0 0 Common adverse events Headache` Fatigue Nausea Diarrhea Upper respiratory tract infection Dizziness Insomnia Irritability 11 11 0 4 7 0 0 0 18 11 11 21 14 7 0 0 33 30 26 4 7 15 19 15 Laboratory abnormalities ALT > 3 x ULN Total bilirubin > 3 x ULN Hemoglobin 8-10 g/dl 0 0 0 0 4 0 0 0 4 SURVEYOR-I-II Part 2 cirrhosis Gane E. Gastroenterology 2016;151:651-9
SURVEYOR-I & II study Part 2 in cirrhosis: glecaprevir + pibrentasvir in genotypes 1 or 3 Summary In this phase 2 study, the once-daily combination of Glecaprevir and Pibrentasvir for 12 weeks is sufficient to achieve high rates of SVR12in patients with genotype 1 or genotype 3 infection and compensated cirrhosis Adverse events were mostly mild in severity Potential for a pangenotypic therapy without RBV co-administration SURVEYOR-I-II Part 2 cirrhosis Gane E. Gastroenterology 2016;151:651-9