COVID-19 Vaccine Updates and Recommendations - January 2023
New updates regarding COVID-19 vaccine administration and recommendations have been released, including the ability for clinics to create and manage vaccine supplies for children as young as six months. The Western States Scientific Review Workgroup supports the use of Moderna and Pfizer-BioNTech bivalent vaccines for young children. The FDA is scheduled to discuss future vaccination regimens on January 26, 2023. Providers can now submit vaccine redistribution plans after a temporary pause. Stay informed with the latest developments in COVID-19 vaccination strategies.
Uploaded on Nov 15, 2024 | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
DPH Provider Vaccine Office Hours: COVID-19 Vaccine Updates 1/04/2023
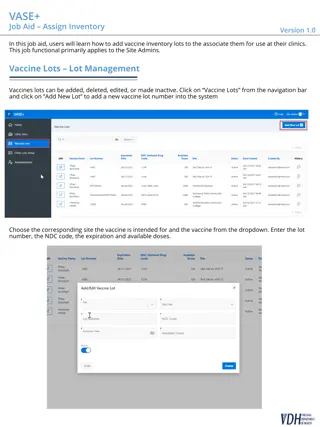
New updates for My Turn Public and Clinic launched Release Highlights Release Highlights Clinic Managers will be able to create clinics and add new vaccine supplies and inventories for Moderna (6 months 5 years) Bivalent Booster and Pfizer (6 months 4 years) Bivalent third dose vaccines. Patients will be able to view updated information about Novavax in the World Health Organization flow while scheduling COVID-19 vaccine appointments. Parents / guardians will be able to schedule Moderna (6 months 5 years) Bivalent Booster and Pfizer (6 months 4 years) Bivalent third dose vaccine appointments for patients 6+ months using the Individual and Group flows. Clinic Managers will be able to view updated Moderna (6 months 5 years) Bivalent Booster and Pfizer (6 months 4 years) Bivalent third dose appointments on the homepage Dashboards. My Turn Clinic My Turn Public Patients will be able to view a new notification regarding the Pfizer Bivalent third dose added on the Home page about scheduling a primary series Pfizer Bivalent third dose vaccine appointment. Clinic Managers will be able to filter, edit, display and resubmit IIS appointments for Moderna (6 months 5 years) Bivalent and Pfizer (6 months 4 years) Bivalent third dose vaccines. Clinic Managers will be able to view a confirmation checkbox, Confirmed patient received last dose of vaccine at least 2 months ago, added under the Patient Background section while editing / resubmitting an IIS record for Moderna (6 months 5 years) Bivalent Booster. Clinic Managers and Vaccine Administrators will be able to filter, single / bulk update and schedule Moderna (6 months 5 years) Bivalent Booster and Pfizer (6 months 4 years) Bivalent third dose vaccine appointments via the Walk-in and Vaccine Administration flow. 2
Western States Scientific Review Workgroup (WSSRW) Recommendation Supports use of Moderna and Pfizer-BioNTech COVID-19 bivalent vaccines in children down to six months of age Bivalent booster doses that more closely match currently circulating variants will help sustain protection against COVID-19-related hospitalizations and deaths FDA recently announced that a meeting of its Vaccines and Related Biological Products Advisory Committee (VRBPAC) is scheduled for January 26, 2023, to discuss future COVID-19 vaccination regimens Western States Scientific Review Workgroup Recommendations: December 23, 2022 3
Redistribution Plans Now Being Accepted CDPH put a pause on accepting/approving redistribution plans for the past several months The plan template has now been updated and CDPH is accepting plans again Providers should submit plans to covidcallcenter@cdph.ca.gov if they are actively redistributing vaccines IMM-1318 Redistribution Vaccine Management Plan (eziz.org) 4
TPR Redistribution of Moderna Bivalent Boosters CDPH is working with AmerisourceBergen, the Third-Party Redistributor (TPR) to provide them with all they need to begin redistribution of the Moderna bivalent boosters Expect to begin redistribution in mid- to late-January 2023 5
KidsVaxGrant 3.0 Target outreach population 978 identified Vaccines for Children (VFC) registered providers who have not enrolled in myCAvax Grant funding opportunities $10,000 for target VFC providers who enroll in myCAvax and attest to placing a minimum of 1 vaccine order within 30- days of award notification $5,000 supplemental grant to those myCAvax enrollees who elect to opt in to enhance/upgrade their electronic health record system Outreach began the week of 12/20 for myCAvax enrollment PHC will begin hosting office hours for myCAvax and grant application information. Inquiries over the holiday were minimal, primarily thanking us for the opportunity to apply to 3.0 and confirming Step 1 is enrollment into myCAvax to become eligible for grant. Tentative grant application launch date Tentative soft launch first week in January 2023 Full open application cycle second week in January 2023 6