Atom Economy and Percentage Yield in Science
Making scientific processes economical and efficient is key in the synthesis of chemical compounds. Atom economy and percentage yield play crucial roles in optimizing production methods to ensure cost-effectiveness and minimal waste generation. Learn how these concepts influence the efficiency of chemical reactions and product synthesis, using examples like the production of Ethanol from Glucose and Ethene.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Atom Economy and Percentage Yield Making science economical as well as scientific !
Atom Economy and Percentage Yield Scientific processes don t come cheap, chemical substances have to be bought, and before that they have to be synthesised. This probably sounds obvious, but much thought needs to be applied to the synthesis of chemical compounds, and if there are several ways to make a certain substance, it stands to reason that, amongst other things ....... 1. The cheapest method is likely to be the preferred one 2. The method that produces the most product will almost certainly be preferred The second point here is going to form the basis of this presentation, atom economy and percentage yield will be explained in the presentation.
Atom Economy and Percentage Yield Let us take a look at a typical process for production of a useful, necessary substance. The synthesis of Ethanol by the hydration of Ethene is one way to produce this industrially and commercially useful product. Ethene is readily produced by the cracking of long chain hydrocarbons and water is of course quite readily available, so this process is relatively economical. The reaction is conducted at moderately high pressure, low temperature and removal of the product on creation favours the forward reaction.
Atom Economy and Percentage Yield Not all reactions are so obliging, and not all are 100% atomically economical like this one, so WHAT exactly do we mean by ATOM ECONOMY? Mrp = Relative Molecular Mass of Required Product Mar = Relative Molecular Mass of ALL Reactants In addition, many reactions will produce by-products that cannot be used for anything, so these are waste and will bring the atom economy of the reaction down.
Atom Economy and Percentage Yield REACTIONS THAT PRODUCE ONLY ONE PRODUCT ARE 100% ATOMICALLY ECONOMICAL
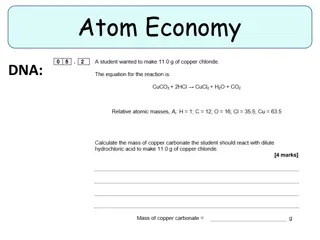
EX 1 - Atom Economy and Percentage Yield Ethanol can be produced by the fermentation of Glucose according to the following equation: RMM of Glucose = (6x12)+(1x12)+(6x16) = 180 RMM of Ethanol = (2x12)+(6x1)+(1x16)= 46 The reaction forms 2 moles of Ethanol per 1 mole of glucose, so the AE is:
EX 2 - Atom Economy and Percentage Yield Nickel can be produced by the reduction of Nickel Oxide with Carbon, producing Nickel metal and Carbon Monoxide. Given the reaction for the equation and RMM of Nickel Oxide = 59+16 = 75 RAM of C = 12:
Atom Economy and Percentage Yield Working out the percentage yield is reasonably straightforward based on your knowledge of moles and molar amounts. Percentage yield is split into two parts: Theoretical Yield - Should be 100% based on molar amounts Actual Yield - This is what we will always get, rarely is a reaction 100% productive. YP= Percentage Yield YA = Actual Yield YT = Theoretical Yield
Atom Economy and Percentage Yield Example - The burning of Magnesium metal in Air (Oxygen) produces Magnesium Oxide according to the equation: Calculate, based on moles and molar amounts, the theoretical yield in grams if 24g of Magnesium was burnt in excess Oxygen. (Mg = 24, O = 16) 1 mole Mg = 24g, so from the equation above you should be able to see that 48g Magnesium reacts with 32g Oxygen to produce 80g Magnesium Oxide (discuss).
Atom Economy and Percentage Yield Let us assume that we started with 0.3g Magnesium (48g would not be practical for a school experiment). What would our THEORETICAL yield be? 0.0125 mol Mg produces 0.0125 mol MgO 0.3g Magnesium is: The reaction is 1:1 with respect to Mg, in other words each mole of Mg should produce a mole of MgO (RMM=40)
Atom Economy and Percentage Yield After our theoretical experiment, we find that we have produced 0.36g MgO....what is the ACTUAL yield as a PERCENTAGE?