Chemical equilibrium
Chemical equilibrium is a state where reactants and products reach a balance in a reaction. Learn about reversible and irreversible reactions, equilibrium constants, and how they affect chemical analysis. Discover the accuracy and applications of volumetric analysis, including titration methods like
3 views • 13 slides
Titration Colour Changes
This collection features various experiments in chemistry involving titration and solutions. It includes procedures such as standardizing hydrochloric acid using sodium carbonate, titrating hydrochloric acid with sodium hydroxide to produce sodium chloride, determining the concentration of ethanoic
0 views • 10 slides
Aspirin Assay by Direct Acid-Base Titration Experiment Overview
Exploring the process of assessing aspirin purity through direct acid-base titration using sodium hydroxide as a standard solution. The experiment includes details on aspirin properties, dosage, acidity, decomposition, and metabolism. Key aspects covered include the aim of the experiment, the princi
5 views • 15 slides
Assay of Ascorbic Acid (Vitamin C) - Method and Procedure
Ascorbic acid, known as Vitamin C, is a crucial organic compound with antioxidant properties. This article discusses the assay of ascorbic acid using a redox titration method with potassium iodate and iodide. The principle, procedure, and calculations involved in determining the concentration of Vit
1 views • 8 slides
Conductometry: An Overview of Measurement and Applications
Conductometry involves measuring the conductivity of a solution to determine its properties. This method includes factors affecting conductivity, conductometric titration, recent developments, and application examples. The process involves using electrodes, primary standard solutions, Wheatstone bri
9 views • 24 slides
Volumetric Analysis Experiment: Standardization of Hydrochloric Acid Using Sodium Carbonate
In this experiment, hydrochloric acid (HCl) is standardized by using standard sodium carbonate (Na2CO3) due to the impurity of HCl. The process involves preparing 0.1N HCl and Na2CO3 solutions, performing a titration to determine the equivalence point, and calculating the concentration of the HCl so
6 views • 28 slides
Belladonna Alkaloids Identification Methods and Analysis
Learn about the qualitative and quantitative analysis methods for identifying belladonna alkaloids. Discover the differences between titration and back-titration, and follow a detailed procedure to quantitatively identify the alkaloids present. Equip yourself with the required equipment and reagents
0 views • 14 slides
Planning Your Flow Cytometry Experiment: Building a Staining Panel
For successful flow cytometry experiments, it is crucial to plan your staining panel carefully by selecting appropriate markers and antibodies. Determine the goal of your experiment, research historical data for similar experiments, and choose markers specific to your cell type. Utilize resources fo
0 views • 19 slides
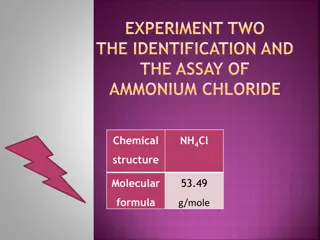
Identification and Assay of Ammonium Chloride: Experiment Insights
Explore the identification and assay process of Ammonium Chloride (NH4Cl), a weak inorganic acid commonly used in various applications. Discover its chemical properties, synthesis, reactivity, identification tests, and details of a titration process using NaOH and phenolphthalein as indicators.
0 views • 16 slides
Chemical Analysis and Redox Reactions in Chemistry
Iron(II) ethanoate concentration determination using redox titration with cerium(IV) sulfate, balanced redox equations for manganate(VII) ion oxidizing iron(II) ion, and calculations of iron percentage in samples using titration with potassium manganate(VII). Molarities and concentrations are calcul
1 views • 6 slides
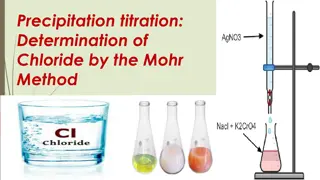
Understanding Precipitation Titration in Analytical Chemistry
Precipitation titration is a method where a precipitate is formed by converting one reacting species. An example is the estimation of chloride using AgNO3, where AgCl is precipitated. The titration is based on the formation of precipitates, and suitable conditions, indicators, and methods are vital
0 views • 30 slides
Overview of Ammonium Chloride: Properties, Preparation, and Uses
Ammonium Chloride, with the formula NH4Cl, is a compound containing not less than 99.5% NH4Cl. It is prepared commercially by neutralizing ammonia with hydrochloric acid or treating ammoniacal gas liquors with lime. The compound is essential for maintaining acid-base equilibrium, acts as an expector
2 views • 4 slides
Understanding Milk Freshness and Quality Control
Milk freshness is crucial for maintaining optimal nutrient composition and preventing excessive acidity due to microbial activity. Various tests and analyses are conducted to assess the freshness and appropriateness of milk for further processing, with titration methods being a common approach for m
2 views • 7 slides
Determination of Acetic Acid Content in Vinegar Experiment
The experiment aims to measure the total acid concentration in a specific brand of vinegar through a titration process using NaOH solution. The procedure involves titrating the vinegar solution with NaOH until a pink color appears, calculating the concentration of acetic acid, and determining the pe
2 views • 8 slides
Back Titration in Analytical Chemistry
Back titration is a technique used in analytical chemistry to determine the concentration of an analyte by reacting it with an excess of another reagent first, followed by titration of the excess reactant. This method is especially useful in cases where direct titration endpoints are difficult to di
2 views • 14 slides
Standardisation of Sodium Hydroxide Solution Experiment
The experiment focuses on the standardisation of sodium hydroxide solution using potassium hydrogen phthalate by titration method. It discusses the types of standard solutions, criteria of primary standards, and the difference between standardisation and titration methods. The objective is to determ
1 views • 26 slides
Sodium Benzoate: Properties, Applications, and Pharmaceutical Uses
Sodium benzoate is a white crystalline powder with various physical and chemical properties. It is commonly used as a preservative in pharmaceutical formulations, cough preparations, and cosmetic products. Additionally, it has pharmaceutical applications in treating urea cycle disorders and schizoph
0 views • 9 slides
Understanding Direct Titration in Analyzing Soil Composition
Exploring the concept of direct titration in soil analysis, focusing on its role in determining the concentration of compounds such as calcium and magnesium ions. This method involves the direct reaction between the unknown compound and a known compound, without the need for excess reagents. Learn h
1 views • 34 slides
Understanding Acid-Base Titration in Chemistry
Acid-base titration is a quantitative technique used to determine the concentration of an unknown acid or base solution. By neutralizing the solution with the opposite component and reaching the equivalence point, students can calculate the concentration using stoichiometry principles. This method d
1 views • 24 slides
Quantitative Estimation of Metal Ions in a Mixture
Dr. Saadia Rashid Tariq explains the quantitative estimation of copper(II), calcium(II), and chloride in a mixture. The process involves iodometric titration for copper(II), complexometric titration for calcium(II), and gravimetric estimation for chloride. Detailed procedures, reactions, requirement
1 views • 8 slides
Redox Titration: Potassium Permanganate with Iron(II) Salt Assay
Ferrous sulfate (FeSO4.7H2O) is used in medical treatments for iron deficiency. This article discusses the redox titration process involving potassium permanganate and ferrous sulfate, along with the chemical principles, procedure, and calculations involved. Potassium permanganate is a powerful oxid
5 views • 7 slides
Determination of Chloride by Mohr Method
Precipitation titration is a volumetric method used for determining chloride ions. Mohr's method involves reacting alkaline or alkaline earth chlorides with silver nitrate in the presence of a potassium chromate indicator. The endpoint of the titration is signaled by the appearance of red silver chr
0 views • 9 slides
Understanding Acid-Base Titration in Chemistry
Acid-base titration is a quantitative technique used to determine the concentration of unknown acids or bases. Through neutralization reactions, the concentration of a solution can be calculated based on the stoichiometry of the reaction. This method dates back to the late 18th century and is crucia
0 views • 24 slides
Assay of Aspirin by Indirect Acid-Base Titration
Indirect titration, also known as residual titration, is an analytical technique used to determine the weight of an unknown sample by employing excess standard solution. In the case of aspirin, which is a weak acid undergoing slow hydrolysis, back titration with NaOH and HCl is utilized to overcome
1 views • 13 slides
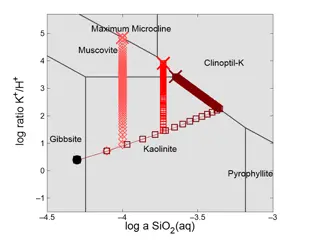
Understanding Reaction Path Traces in GWB12
Visualizing reaction path traces in GWB12 using Act2 or Tact diagrams helps in analyzing how mineral stability and fluid chemistry evolve during reactions like K-feldspar titration. By overlaying multiple traces, customizing markers and lines, users can explore the impact of different silica polymor
0 views • 6 slides
Buprenorphine Induction and Maintenance Guidelines
Guidelines for buprenorphine induction and maintenance for patients dependent on short-acting or long-acting opioids. Covers microdosing, home vs. office induction, managing withdrawal symptoms, dose titration, patient education, and follow-up protocols. Includes information on assessing withdrawal,
0 views • 17 slides
Science Exemplars: A Companion to Graphic Organisers Resource Book
Explore science exemplars covering topics such as water treatment, plant reproduction, titration, digestion stages, organism organisation, scientific method, alimentary canal, microscopic study of plant cells, chemistry concepts, growing bacteria, and oxygen gas production. These exemplars provide p
0 views • 55 slides
Understanding Acid-Base Titration in Analytical Chemistry
Analytical chemistry involves analyzing material samples to determine their chemical composition. Acid-base titration is a technique used to find the concentration of an unknown solution by reacting it with a known concentration solution. This process, involving neutralization reactions and pH indic
0 views • 4 slides
Estimation of Free CO2 in Water Sample for B.Sc. III Year Practical
The practical session focuses on estimating free CO2 in a water sample using titration methods with NaOH and Na2CO3. Students learn the process of converting carbonic acid to neutral sodium bicarbonate to determine the presence of free CO2. Procedures, reagents, observations, and calculations are de
0 views • 6 slides
Determination of Bicarbonate in Blood Using Back Titration
Back titration is an analytical chemistry technique used to determine the concentration of an analyte, such as bicarbonate in blood. This method involves reacting the analyte with an excess reagent, followed by back-titrating the remaining excess and relating it to the original sample's concentratio
0 views • 21 slides
Enhancing Flow Cytometry Practices for Accurate Data Analysis
Explore the realm of forensic flow cytometry with emphasis on improving data quality through proper titration and blocking techniques. Learn to identify and address common flow cytometry issues in various experimental cases to ensure reliable and reproducible results. Dive into practical rules and m
0 views • 39 slides
Management of Anti-e Late Sensitization in Pregnancy: A Case Study
A detailed case study involving a pregnant patient with anti-e late sensitization, suspected HDFN, and challenging component selection decisions. The patient's history, serology results, obstetric background, and sequence of events leading to an urgent transfer of the infant to a specialized hospita
0 views • 15 slides
Acid-Base Titration Lab Procedure & Calculations
Conduct an acid-base titration experiment to determine the molarity of an unknown NaOH solution using HCl as the titrant. Follow the outlined steps, record data, perform calculations, and analyze the results to understand the principles of this chemical reaction. Learn about indicators, mole ratios,
0 views • 7 slides
Understanding Volumetric Analysis in Chemical Experiments
Chemical analysis is crucial in studying material composition. Volumetric analysis, a key procedure, involves measuring reaction volumes in solutions to determine substance concentrations. This method utilizes titration and different chemical reaction types like acid-base and precipitation methods.
0 views • 17 slides
Understanding the Titration of Weak Acids and Amino Acids
Titration is a crucial technique used to determine the properties of weak acids and amino acids. This process involves calculating pH values, degree of ionization, and understanding the ionization equilibrium of different acid-base systems. Various examples, including glycine hydrochloride, isoelect
0 views • 15 slides
Clinical Cases of Diabetes Management: Insights and Recommendations
Explore three detailed cases of patients with type 2 diabetes mellitus, their current treatment regimens, complications, and potential adjustments to improve glycemic control. Recommendations for treatment modifications, including insulin initiation, titration options, and lifestyle changes. Enhance
0 views • 23 slides
Telehealth in Heart Failure Management: Improving Outcomes through Digital Solutions
Research indicates that telehealth programs play a significant role in managing chronic heart failure, leading to reduced all-cause mortality, decreased hospitalizations, and improved quality of care. Studies highlight the effectiveness of telehealth in monitoring symptoms, enhancing patient outcome
0 views • 21 slides
Assay of Ferrous Sulfate (FeSO4.7H2O) by Redox Titration Experiment
This experiment involves determining the weight and weight percentage of an unknown sample of FeSO4.7H2O through a redox titration using potassium permanganate solution. Ferrous sulfate, a chemical compound used in medical treatments, is oxidized to ferric sulfate in the presence of sulfuric acid. T
0 views • 9 slides
Assay of NaOH Solution - Lab Procedure for Determining Sodium Hydroxide Content
This essay discusses the assay of NaOH solution, focusing on the two-step titration process involving NaOH and HCl for determining the concentration of NaOH. Detailed procedures, chemical factors, and calculations for both titration steps are explained in depth. The analysis also includes the ration
0 views • 10 slides
Analyzing Precipitation Titrations and Spectroscopy in Chemistry Lecture
Exploring the concepts of precipitation titrations and spectroscopy in a chemistry lecture covering topics such as titration curves, sharpness at equivalence points, and titration of mixtures. The lecture delves into examples like the titration of Hg2^2+ by CrO4^2-, emphasizing the three regimes bef
0 views • 17 slides