Volumetric Analysis Experiment: Standardization of Hydrochloric Acid Using Sodium Carbonate
In this experiment, hydrochloric acid (HCl) is standardized by using standard sodium carbonate (Na2CO3) due to the impurity of HCl. The process involves preparing 0.1N HCl and Na2CO3 solutions, performing a titration to determine the equivalence point, and calculating the concentration of the HCl solution. This practical chemistry procedure is essential for accurate volumetric analysis in medical physics.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
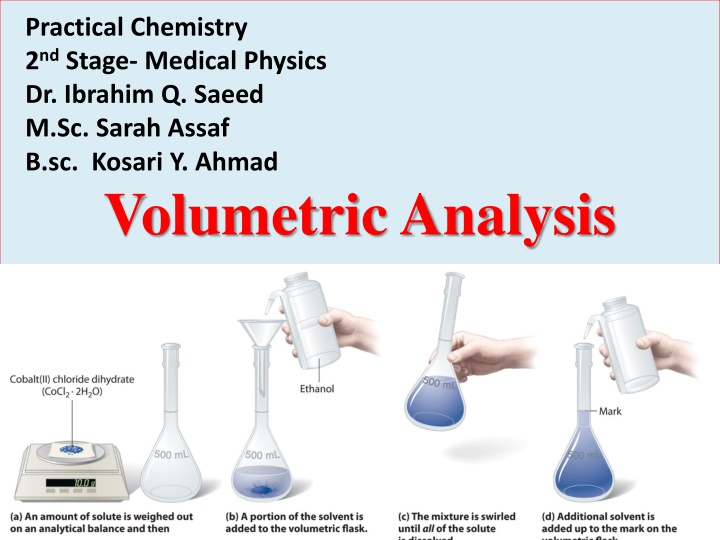
Practical Chemistry 2nd Stage- Medical Physics Dr. Ibrahim Q. Saeed M.Sc. Sarah Assaf B.sc. Kosari Y. Ahmad Volumetric Analysis
Experiment No.1 Preparation and standardization of 0.1N hydrochloric acid (HCl) using standard sodium carbonate (Na2CO3)
Hydrochloric acid (HCl) is not standard substance; Because HCl cannot be obtained at a very high purity and HCl does not have a high molecular mass. therefore it must standardize by using standard sodium carbonate (Na2CO3). Na2CO3 + 2 HCl 2 NaCl + H2CO3 2 NaCl + CO2 + H2O
Preparation of solutions 1- Prepare 0.1N Na2CO3 in 250mL of distilled water Na2CO3 is a solid substance. N eq.wt V (ml) F.wt of Na2CO3 2 1 Wt = eq.wt = 1000 2 23 + 1 12 + 3 16 0.1 53 250 eq.wt = Wt = 2 1000 106 = 53 g/mol eq.wt = 2 Wt = 1.325 g Dissolve 1.325 g Na2CO3 in approximately 250 ml of distilled water to obtain 0.1N Na2CO3.
2- Prepare 0.1N HCl in 250mL of distilled water HClis a liquid substance. Sp.gr = 1.19 g/ml %HCl = 37% Sp.gr % 1000 F.wt of HCl eq.wt = N = 1 eq.wt 1 1 + 1 35.5 37 100 1000 eq.wt = 1.19 1 N = 36.5 eq.wt = 36.5 g/mol N = 12 eq/L
no. of milliequivalence after dilution no. of milliequivalence before dilution = (N2 V2)dilu. (N1 V1)conc.= 12 V1 = 0.1 250 0.1 250 12 = 2.08 ml = 2.1 ml V1= Take 2.1 ml of concentrated HCl with a pipet, and dilute to 250 ml with distilled water in a 250ml-volumetric flask to obtain approximately 0.1 N HCl.
Procedure: 10ml of HCl (0.1N) 0.1 N Na2CO3solution 2-3 drops of methyl orange 10 ml of HCl solution 2 drops of methyl orange The Colour changed from Red - to - yelow (1) (2) (3)
Calculation At the equivalence point no. of milliequivalence of HCl no. of milliequivalence of Na2CO3= (N1 V1) Na2CO3= (N2 V2)HCl 0.1 Vaverage = N2 10 0.1 Vaverage 10 = ( ) eq/L HCl N2=
Experiment No. 2 Preparation and standardization of approximately 0.1N sodium hydroxide (NaOH) using standardized hydrochloric acid (HCl)
Sodium hydroxide are always coated with thin layer of sodium carbonate as a result of reaction of hydroxide with atmospheric carbon dioxide (CO2). Na2CO3 + H2O 2 NaOH + CO2 Pure sodium hydroxide cannot be obtained; so that a standard solution of NaOH cannot be prepared by dissolving a known weight in a definite volume of water. Therefore sodium carbonate should remove from sodium hydroxide before preparation of the solution. Several methods are available for this purpose:-
1- Ba(OH)2 method: A slight excess of barium hydroxide is added to the solution of NaOH to precipitate carbonate as BaCO3 (precipitation), then filtered the solution to remove the precipitate (Filtration). BaCO3 + 2 NaOH Ba(OH)2 + Na2CO3
2-Anion exchange method It involves passing NaOH solution through a column of a strongly basic anion exchanger which has higher affinity to carbonate anion CO3-2 than for hydroxide OH-. The carbonate is removed and replaced by OH- of the anion exchanger. NaOH solution CO3-2 CO3-2 -Form OH- -Form Anion exchange NaOH solution Free from CO3-2
Preparation of solutions Prepare 250mL aqueous solution of an approximate 0.1N HCl. Prepare 250mL aqueous solution of an approximate 0.1N NaOH Reaction: NaOH + HCl NaCl + H2O
Preparation of 250mL solution of approximate 0.1N NaOH Dissolve 1.00 gm of NaOH in small amount of water then complete to 250 ml with distilled water to obtain 0.1N NaOH solution.
Experiment No. 3 Preparation and standardization of approximately 0.1N acetic acid solution (CH3COOH) by using standardized sodium hydroxide (NaOH)
Acetic acid (CH3COOH) also known as ethanoic acid, (Ka = 1.8 10-5 at 25 C) and partially dissociated in an aqueous solution. Glacial acetic acid is a pure and water-free acetic acid. It is a colorless liquid that absorbs water from the environment (hygroscopic), freeze at 16.5 C and change to a colorless crystalline solid. Acetic acid can be detected by its characteristic smell which has a distinctive (clear) pungent (hot) odour.
Concentrated acetic acid is corrosive and therefore must be handled with appropriate care, since it can cause skin burns and permanent eye damage. These burns may not appear until hours after exposure. The titration of CH3COOH against NaOH solution is a titration of weak acid against strong base.
The solution at equivalence point not neutral, but slightly alkaline, the calculated pH at equivalence point is 8.72. In this experiment, (methyl orange) or (methyl red) cannot be used because they change their color at a much lower pH (acidic medium), i.e. the color is changed before the equivalence point. Therefore, phenolphthalein (ph.ph) is used as indicator because its color changes in the alkaline region at a pH range of (8-9.6). Preparation of solutions: 1- Prepare 0.1 N NaOH in 250 mL of distilled water. 2- Prepare approximately 0.1 N CH3COOH in 250 mL of distilled water.
Prepare approximately 0.1 N CH3COOH in 250 mL of distilled water. Sp.gr = 1.049 , % = 98% , Formula weight = 60 g/mole. Sp.gr % 1000 F.wt of CH3COOH eq.wt = N = 1 eq.wt 1.049 98 2 12 + 4 1 + 2 16 1000 eq.wt = 100 1 N = 60 eq.wt = 60 g/mol N = 17.13 eq/L (N1 V1)conc. (N2 V2)dil. = 0.1 250 17.13 V1 = = 1.459 1.5 mL 17.13 V1 0.1 250 = You must take 1.5 ml of concentrated CH3COOH with a pipet, and dilute to 250 ml with distilled water in a 250ml-volumetric flask to obtain approximately 0.1 N CH3COOH.
Procedure: 1. Pipet 5 ml of acetic acid solution into a conical flask. 2. Add 2-3 drops of phenolphthalein indicator. The solution will be colorless. 3. Titrate the solution with the standardized NaOH solution from the burette until the color of solution changes into pink. Repeat the titration three (3) times. V CH3COOH (ml) 5 5 5 VNaOH(ml) V1 V2 V3 V1 + V2 + V3 3 V average = = (Y) ml NaOH
5 ml of 0.1 N NaOH solution CH3COOH (0.1N) 2-3 drops of phenolphthalein (ph.ph.) 5 ml of CH3COOH solution 2-3 drops of (ph.ph.) The Colour changed from collorless to pink (1) (2) (3)
Calculation: At the equivalence point no. of milliequivalence of CH3COOH no. of milliequivalence of NaOH = (N1 V1)NaOH = (N2 V2) CH3COOH N2 5 0.1 Vaverage = N2 5 0.1 Y = 0.1 Y = ( ) eq/L CH3COOH N2= 5
Experiment No. 4 Application of Neutralization Titration (Acid Base Titration): Determination of Acetic Acid in Vinegar Vinegar is a combination of acetic acid and water made by a two-step fermentation process. First, yeast feed on the sugar or starch of any liquid from a plant food such as fruits, whole grains, potatoes, or rice. This liquid ferments into alcohol. The alcohol is then exposed to oxygen and the acetic acid bacteria Acetobacter to ferment again over weeks or months, forming vinegar. Most commercial vinegars contain about 5% by weight of acetic acid. Procedure Take 10 ml of vinegar and dilute to 250 ml.
10 ml of vinegar 0.1 N NaOH solution 2-3 drops of phenolphthalein (ph.ph.) 10 ml of vinegar 2-3 drops of (ph.ph.) The Colour changed from collorless to pink (1) (2) (3)
Calculation (N) of acetic acid in 250 ml vinegar (N V)NaOH = (N V)A.A. (0.1 Vaverage)NaOH = (N 10)A.A. N = X eq./L (Normal) (Acetic acid in 250 ml vinegar) Calculation (N) of acetic acid in 10 ml vinegar (N V)Before dil. = (N V)After dil. (N 10)Before dil. = (X 250)After dil. N = Y eq./L (Normal) (Acetic acid in 10 ml vinegar)
Y Wt.= B g (Acetic acid in 10 ml vinegar)