Titration Colour Changes
This collection features various experiments in chemistry involving titration and solutions. It includes procedures such as standardizing hydrochloric acid using sodium carbonate, titrating hydrochloric acid with sodium hydroxide to produce sodium chloride, determining the concentration of ethanoic
0 views • 10 slides
Aspirin Assay by Direct Acid-Base Titration Experiment Overview
Exploring the process of assessing aspirin purity through direct acid-base titration using sodium hydroxide as a standard solution. The experiment includes details on aspirin properties, dosage, acidity, decomposition, and metabolism. Key aspects covered include the aim of the experiment, the princi
5 views • 15 slides
Assay of Ascorbic Acid (Vitamin C) - Method and Procedure
Ascorbic acid, known as Vitamin C, is a crucial organic compound with antioxidant properties. This article discusses the assay of ascorbic acid using a redox titration method with potassium iodate and iodide. The principle, procedure, and calculations involved in determining the concentration of Vit
1 views • 8 slides
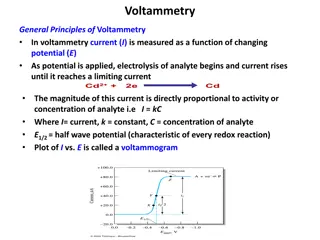
Understanding Voltammetry: Principles and Applications
In voltammetry, current is measured as a function of changing potential. The magnitude of current is directly proportional to the activity or concentration of the analyte. A voltammogram is plotted between current and potential, showing the characteristic half-wave potential. The process involves a
6 views • 76 slides
Belladonna Alkaloids Identification Methods and Analysis
Learn about the qualitative and quantitative analysis methods for identifying belladonna alkaloids. Discover the differences between titration and back-titration, and follow a detailed procedure to quantitatively identify the alkaloids present. Equip yourself with the required equipment and reagents
0 views • 14 slides
Advancements in Chemical Mechanisms for Air Quality Management
Daniel Jacob and team have been enhancing chemical mechanisms in the GEOS-Chem model to support US air quality management. Ongoing work includes developing new mechanisms for aromatic VOCs, tropospheric halogens, mercury redox, adaptive mechanism reduction, machine learning applications, and unifica
0 views • 19 slides
Chemical Analysis and Redox Reactions in Chemistry
Iron(II) ethanoate concentration determination using redox titration with cerium(IV) sulfate, balanced redox equations for manganate(VII) ion oxidizing iron(II) ion, and calculations of iron percentage in samples using titration with potassium manganate(VII). Molarities and concentrations are calcul
1 views • 6 slides
Understanding Redox Reactions in Chemistry
Salts can be prepared through redox reactions involving metals and acids. This interactive lesson covers oxidation numbers, identifying oxidized and reduced elements, and explaining electron transfer in redox reactions. Examples include reactions of aluminum with sulfuric acid and magnesium with cop
2 views • 12 slides
Understanding Precipitation Titration in Analytical Chemistry
Precipitation titration is a method where a precipitate is formed by converting one reacting species. An example is the estimation of chloride using AgNO3, where AgCl is precipitated. The titration is based on the formation of precipitates, and suitable conditions, indicators, and methods are vital
0 views • 30 slides
Overview of Ammonium Chloride: Properties, Preparation, and Uses
Ammonium Chloride, with the formula NH4Cl, is a compound containing not less than 99.5% NH4Cl. It is prepared commercially by neutralizing ammonia with hydrochloric acid or treating ammoniacal gas liquors with lime. The compound is essential for maintaining acid-base equilibrium, acts as an expector
2 views • 4 slides
Dakin Rearrangement in Organic Chemistry: Mechanism and Positional Effects
The Dakin Rearrangement, also known as Dakin oxidation, is an organic redox reaction involving hydroxylated phenyl aldehydes or ketones reacting with hydrogen peroxide to form benzenediols and carboxylates. The mechanism includes nucleophilic addition, [1,2]-aryl migration, and final product formati
1 views • 9 slides
Understanding Milk Freshness and Quality Control
Milk freshness is crucial for maintaining optimal nutrient composition and preventing excessive acidity due to microbial activity. Various tests and analyses are conducted to assess the freshness and appropriateness of milk for further processing, with titration methods being a common approach for m
2 views • 7 slides
Back Titration in Analytical Chemistry
Back titration is a technique used in analytical chemistry to determine the concentration of an analyte by reacting it with an excess of another reagent first, followed by titration of the excess reactant. This method is especially useful in cases where direct titration endpoints are difficult to di
2 views • 14 slides
Standardisation of Sodium Hydroxide Solution Experiment
The experiment focuses on the standardisation of sodium hydroxide solution using potassium hydrogen phthalate by titration method. It discusses the types of standard solutions, criteria of primary standards, and the difference between standardisation and titration methods. The objective is to determ
1 views • 26 slides
Understanding Enemark-Feltham Notation in Iron-Nitrosyl Complexes
Iron-Nitrosyl complexes are redox non-innocent, with NO exhibiting multiple redox states. Enemark-Feltham Notation helps in determining metal-ligand interactions and oxidation states. Detailed information on NO ligands, bonding characteristics, and methods for analyzing iron-NO systems are discussed
0 views • 6 slides
Understanding Direct Titration in Analyzing Soil Composition
Exploring the concept of direct titration in soil analysis, focusing on its role in determining the concentration of compounds such as calcium and magnesium ions. This method involves the direct reaction between the unknown compound and a known compound, without the need for excess reagents. Learn h
1 views • 34 slides
Chemical Reactions and Energy Transfers
Understanding chemical reactions involving thermal decomposition of metal carbonates, identifying exothermic and endothermic reactions based on energy transfers, and recognizing oxidation and reduction in redox reactions.
1 views • 5 slides
Understanding Soil Chemistry and Redox Reactions in Environmental Chemistry
Soil chemistry plays a crucial role in sustaining healthy soils by influencing nutrient availability through oxidation and reduction processes. Redox reactions in soil are impacted by factors like oxygen content and water presence, affecting nutrient supplies. The redox status of soil reflects its n
1 views • 92 slides
Understanding Acid-Base Titration in Chemistry
Acid-base titration is a quantitative technique used to determine the concentration of an unknown acid or base solution. By neutralizing the solution with the opposite component and reaching the equivalence point, students can calculate the concentration using stoichiometry principles. This method d
0 views • 24 slides
Quantitative Estimation of Metal Ions in a Mixture
Dr. Saadia Rashid Tariq explains the quantitative estimation of copper(II), calcium(II), and chloride in a mixture. The process involves iodometric titration for copper(II), complexometric titration for calcium(II), and gravimetric estimation for chloride. Detailed procedures, reactions, requirement
1 views • 8 slides
Redox Titration: Potassium Permanganate with Iron(II) Salt Assay
Ferrous sulfate (FeSO4.7H2O) is used in medical treatments for iron deficiency. This article discusses the redox titration process involving potassium permanganate and ferrous sulfate, along with the chemical principles, procedure, and calculations involved. Potassium permanganate is a powerful oxid
5 views • 7 slides
Determination of Chloride by Mohr Method
Precipitation titration is a volumetric method used for determining chloride ions. Mohr's method involves reacting alkaline or alkaline earth chlorides with silver nitrate in the presence of a potassium chromate indicator. The endpoint of the titration is signaled by the appearance of red silver chr
0 views • 9 slides
Understanding Potentiometry and Electrochemical Cells
Electrochemical cells play a vital role in redox reactions, with potentiometry being a quantitative analysis method based on measuring potentials. These cells consist of two half-cells where reduction and oxidation take place, forming anode and cathode. Reference electrodes like calomel and Ag/AgCl
0 views • 9 slides
Understanding Redox Reactivity and Balancing Equations in Acidic Solutions
Exploring the concept of reactivity in redox reactions using zinc, nickel, and copper, followed by a detailed guide on balancing redox equations in acidic solutions. Learn how to determine oxidation numbers, identify redox atoms, balance charges, and handle oxygen and hydrogen atoms to achieve balan
0 views • 18 slides
Understanding Acid-Base Titration in Chemistry
Acid-base titration is a quantitative technique used to determine the concentration of unknown acids or bases. Through neutralization reactions, the concentration of a solution can be calculated based on the stoichiometry of the reaction. This method dates back to the late 18th century and is crucia
0 views • 24 slides
Assay of Aspirin by Indirect Acid-Base Titration
Indirect titration, also known as residual titration, is an analytical technique used to determine the weight of an unknown sample by employing excess standard solution. In the case of aspirin, which is a weak acid undergoing slow hydrolysis, back titration with NaOH and HCl is utilized to overcome
1 views • 13 slides
Understanding Acid-Base Titration in Analytical Chemistry
Analytical chemistry involves analyzing material samples to determine their chemical composition. Acid-base titration is a technique used to find the concentration of an unknown solution by reacting it with a known concentration solution. This process, involving neutralization reactions and pH indic
0 views • 4 slides
Understanding Electron Transport Chain and ATP Synthesis in Biochemistry
This course delves into the intricacies of electron transport and oxidative phosphorylation in biochemistry, elucidating how NADH and FADH2 are re-oxidized to generate ATP in eukaryotes and prokaryotes. It explores redox potential, oxidation-reduction reactions, and the role of standard redox potent
0 views • 20 slides
Determination of Bicarbonate in Blood Using Back Titration
Back titration is an analytical chemistry technique used to determine the concentration of an analyte, such as bicarbonate in blood. This method involves reacting the analyte with an excess reagent, followed by back-titrating the remaining excess and relating it to the original sample's concentratio
0 views • 21 slides
Understanding Electrochemistry Concepts and Redox Reactions
Explore the fundamentals of electrochemistry, oxidation-reduction reactions, and identification of redox components. Learn about oxidation states, electron transfer, and half-reactions. Dive into examples and visual aids to grasp the concepts easily.
0 views • 42 slides
Understanding Electrochemistry: Redox Reactions, Cells, and Equations
Electrochemistry is a branch of chemistry that involves redox reactions, galvanic cells, standard reduction potentials, balancing redox equations, batteries, corrosion prevention, and electrolysis. Learn about the fundamental principles, examples of redox reactions, and how to balance equations usin
0 views • 61 slides
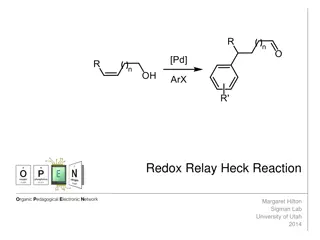
Understanding the Redox-Relay Heck Reaction in Organic Synthesis
The Redox-Relay Heck Reaction is a powerful tool in organic synthesis that allows for the functionalization of olefins with aryl groups. Developed by Sigman and colleagues, this reaction involves a palladium-catalyzed relay controlled by a thermodynamic sink, leading to the formation of aldehydes or
0 views • 6 slides
Redox Conditions and pH Control in a Mountain Watershed: Study in Red Canyon, Wyoming, USA
Exploring redox conditions and trace element concentrations in a semi-arid mountain watershed, this study in Red Canyon, Wyoming, delves into the impact of oxic surface water and anoxic groundwater on trace element cycling. The investigation aims to enhance understanding of seasonal variabilities, f
0 views • 11 slides
Acid-Base Titration Lab Procedure & Calculations
Conduct an acid-base titration experiment to determine the molarity of an unknown NaOH solution using HCl as the titrant. Follow the outlined steps, record data, perform calculations, and analyze the results to understand the principles of this chemical reaction. Learn about indicators, mole ratios,
0 views • 7 slides
Understanding the Titration of Weak Acids and Amino Acids
Titration is a crucial technique used to determine the properties of weak acids and amino acids. This process involves calculating pH values, degree of ionization, and understanding the ionization equilibrium of different acid-base systems. Various examples, including glycine hydrochloride, isoelect
0 views • 15 slides
Assay of Ferrous Sulfate (FeSO4.7H2O) by Redox Titration Experiment
This experiment involves determining the weight and weight percentage of an unknown sample of FeSO4.7H2O through a redox titration using potassium permanganate solution. Ferrous sulfate, a chemical compound used in medical treatments, is oxidized to ferric sulfate in the presence of sulfuric acid. T
0 views • 9 slides
Assay of NaOH Solution - Lab Procedure for Determining Sodium Hydroxide Content
This essay discusses the assay of NaOH solution, focusing on the two-step titration process involving NaOH and HCl for determining the concentration of NaOH. Detailed procedures, chemical factors, and calculations for both titration steps are explained in depth. The analysis also includes the ration
0 views • 10 slides
Analyzing Precipitation Titrations and Spectroscopy in Chemistry Lecture
Exploring the concepts of precipitation titrations and spectroscopy in a chemistry lecture covering topics such as titration curves, sharpness at equivalence points, and titration of mixtures. The lecture delves into examples like the titration of Hg2^2+ by CrO4^2-, emphasizing the three regimes bef
0 views • 17 slides
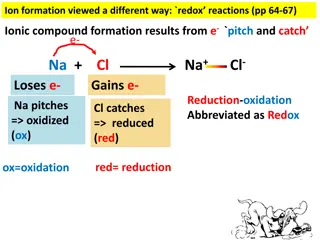
Understanding Redox Reactions and Ionic Compound Formation
Explore the concept of redox reactions through the process of ion formation, where elements lose or gain electrons to create ionic compounds. Learn about oxidation (ox) and reduction (red) in chemical reactions, and how to identify which elements lose or gain electrons based on charge changes. Disco
0 views • 14 slides
Understanding Redox Half Reactions: Assigning Oxidation Numbers and Half-Reactions
In this content, we explore assigning oxidation numbers to elements in compounds such as HClO, HClO2, HClO3, and HClO4. We then delve into the Haber Process to understand redox reactions. The concept of oxidation and reduction, as well as the significance of electrons in these reactions, is illustra
0 views • 20 slides