Fuel Chemistry in Internal Combustion Engines
Fuel chemistry knowledge is crucial for internal combustion engines for various reasons such as determining air-fuel ratios, ensuring efficient combustion, preventing detonation, and optimizing power output. Balancing chemical equations, calculating specific volume for mixtures, and analyzing first
10 views • 19 slides
Maxwell Equations in Thermodynamics
In thermodynamics, Maxwell equations are derived using Euler's reciprocity relation. They involve characteristic functions such as internal energy, free energy, enthalpy, and Gibbs free energy, along with parameters like temperature, entropy, pressure, and volume. These equations form the foundation
1 views • 15 slides
Qualitative Analysis Experiments and Bond Enthalpy
Qualitative tests are conducted on an unknown compound to identify it, followed by an experiment analyzing the rate of hydrolysis in different haloalkanes. Experiments involving silver nitrate and haloalkanes provide insights into the bond enthalpies of carbon-halogen bonds. Suggestions on modifying
0 views • 7 slides
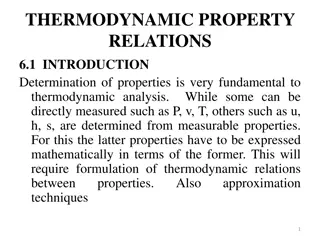
Fundamentals of Thermodynamic Property Relations
Determining thermodynamic properties is crucial for analysis, involving direct and derived properties expressed through formulations and relations. Partial derivatives, fundamental relations like the 1st and 2nd laws, and functions such as enthalpy, Helmholtz, and Gibbs are explored. Maxwell relatio
5 views • 56 slides
Calorimetry and Enthalpy Changes in Chemistry
This content explores various concepts related to calorimetry, enthalpy changes, and Hess's Law in the field of chemistry. It covers topics such as energy released in combustion reactions, heat capacity, phase changes, and the application of Hess's Law in determining enthalpy changes. Enthalpy value
1 views • 17 slides
Thermodynamic Principles of Polymers in Solution: Flory-Huggins Theory and Macromolecular Solutions
Understanding the thermodynamic behavior of polymers in solution is crucial in various industrial and scientific applications. The Flory-Huggins theory provides insights into athermal macromolecular solutions, heat of mixtures, interaction parameters, and the concept of good vs. mediocre solvents. T
1 views • 77 slides
Performance Limits of Accelerator Dipole and Quadrupole for Muon Collider
Utilizing Python code, the study analyzes the limits of accelerator dipole and quadrupole for the Muon Collider. Analytic formulas are implemented to assess the behavior of these components based on critical current density, operating temperatures, and superconductor materials. The study explores li
0 views • 17 slides
Phase Transformations and Latent Heat Equation in Statistical Mechanics
In this informative piece by Dr. N. Shanmugam, Assistant Professor at DGGA College for Women, Mayiladuthurai, the concept of phase transformations in substances as they change states with temperature variations is explored. The latent heat equation is discussed along with definitions of fusion, vapo
3 views • 22 slides
General Chemistry Chem 101 Homework Review
This homework assignment covers various topics in general chemistry, including gas expansion work calculations, heat transfer, enthalpy of formation, and specific heat calculations. Each question presents a different scenario for students to apply their knowledge and problem-solving skills. The ques
0 views • 7 slides
Dimensionless Velocity Triangles and Relations in Turbomachinery
Explore the concept of dimensionless velocity triangles for passive and active machines, power coefficients, Gibbs equation, static enthalpy coefficients, degree of reaction based on enthalpy and pressure changes, and Euler's turbine formula in kinematic form. Gain insights into the relationships an
1 views • 17 slides
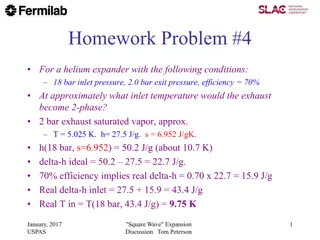
Helium Expander Inlet Temperature Analysis
Analyzing a helium expander operating at 18 bar inlet pressure and 2.0 bar exit pressure with a 70% efficiency to determine the inlet temperature at which the exhaust transitions to a two-phase state. The process involves calculations based on enthalpy values and real temperature at the inlet. Exper
0 views • 18 slides
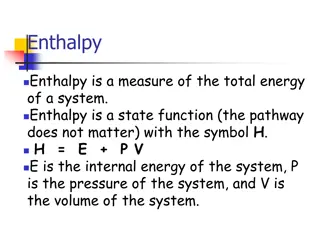
Enthalpy and Heat Capacity in Chemistry
Enthalpy is a measure of total energy in a system, represented as H = E + P.V. Heat at constant pressure relates to enthalpy changes. Calorimetry and heat capacity help measure and understand heat in chemical reactions. Specific heat capacity and molar heat capacity play key roles in determining ene
1 views • 16 slides
Thermochemistry: Heat, Physical Changes, and Methods of Measurement
Thermochemistry delves into heat, physical changes, and various methods of measuring enthalpy, including calorimetry, Hess's Law, standard enthalpy of formation, and bond energies. Specific heat capacity is crucial for determining the amount of heat absorbed or released during a temperature change.
1 views • 27 slides
Basics of Thermochemistry and Energy Changes in Chemistry
Thermochemistry in chemistry involves energy change, the First Law of Thermodynamics, and concepts like heat, work, and internal energy. It explores calculations of heat exchange using calorimeters, Enthalpy Change, Enthalpy of Formation, and more. Energy basics cover the importance of energy change
2 views • 53 slides
Thermochemistry: Heat, Temperature, and Enthalpy
Thermochemistry is the study of heat energy associated with chemical and physical changes. It involves understanding concepts like heat transfer, temperature, enthalpy, and specific heat capacity. By exploring these principles, we can analyze heat changes in systems and calculate energy requirements
0 views • 11 slides
Exothermic and Endothermic Reactions in Chemistry
In chemistry, it's essential to differentiate between exothermic and endothermic reactions based on the direction of heat flow. Exothermic reactions release heat to the surroundings, while endothermic reactions absorb heat to proceed. Through examples like the combustion of propane and the formation
1 views • 21 slides
Nitrogen Family: Properties, Characteristics, and More
Delve into the Nitrogen Family, consisting of two nonmetals, metalloids, and a metal. Learn about their unique properties, electron configurations, atomic symbols, and ionic radii. Discover how ionization enthalpy and electronegativity vary within the group, shedding light on the behavior of these e
0 views • 11 slides
Exploring Thermochemistry and Heat Energy Transfer
Thermochemistry involves the transfer of heat energy during chemical reactions and physical changes. Understanding concepts like enthalpy, heat capacity, and energy flow is crucial. The high heat capacity of water plays a vital role in moderating temperature changes in the environment. The law of co
1 views • 16 slides
Standard Molar Enthalpies of Formation
Formation reactions involve substances being created from elements in their standard states, with the enthalpy change known as the standard molar enthalpy of formation (Hf). This enthalpy represents the energy released or absorbed when one mole of a compound is formed from its elements in their stan
2 views • 13 slides
Thermodynamics of Materials: Experimental Verification and Entropy Analysis
Verification of the Third Law of Thermodynamics through the study of phase transitions in elements like sulfur, exemplifying the relationship between heat capacity, enthalpy, entropy, and the Third Law. Experimental data on entropy changes and molar entropies, as well as insights into the behavior o
0 views • 24 slides
Trapping Modelling in MatCalc: Understanding Gibbs Energy and Configurational Entropy
Trapping modelling in MatCalc involves studying the entrapment of atoms on lattice defects like solute atoms and dislocations. This process affects the system's energy and concentration, with parameters such as trapping enthalpy, nominal composition, and molar volume playing vital roles. By consider
0 views • 21 slides
Bond Enthalpy in Chemistry
Bond enthalpy represents the energy needed to break chemical bonds and is crucial in understanding the strength of these bonds. Explore concepts like bond length, potential energy diagrams, and bond energy calculations.
0 views • 15 slides
Biochemistry
Bioenergetics is the quantitative study of energy transformations in living cells, governed by the laws of thermodynamics. Living systems constantly exchange energy and matter with their surroundings to maintain order while operating within the principles of energy conservation and increasing entrop
0 views • 23 slides
Thermodynamics: Spontaneity, Entropy, and Free Energy
Definitions of spontaneous processes, entropy, enthalpy, and free energy in thermodynamics. Exploring the laws of thermodynamics, entropy changes in chemical reactions, and Gibbs free energy. Understand factors affecting entropy increase and calculations involved.
0 views • 9 slides
Bomb Calorimetry: Measuring Heat of Organic Materials
Bomb calorimetry is a technique used to measure the heat of combustion for organic materials. It involves saturating the material with oxygen in a sealed container (the bomb) and igniting it to record the enthalpy change. The process converts carbon molecules to carbon dioxide, hydrogen to water, an
0 views • 6 slides
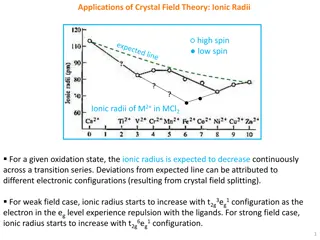
Crystal Field Theory: Applications in Transition Metal Ion Properties
Crystal Field Theory explains trends in ionic radii, lattice energy, and hydration enthalpy in transition metal ions. The theory also describes the Jahn-Teller Effect and its types of distortions.
0 views • 8 slides
Hess's Law and Enthalpy Calculations
Learn how to apply Hess's Law to calculate enthalpy changes in chemical reactions. Discover the principles behind Hess's Law, its application in determining enthalpy changes, and verification methods through different routes. Explore diagrammatic representations and simultaneous equations illustrati
0 views • 16 slides
When I Use a Word - Calorific Value and Energy Release
In this content, Lewis Carroll's quote on word meanings is juxtaposed with information on calorific value, energy release, and combustion reactions. It covers the differences between Higher and Lower Calorific Values, the recovery of latent heat in modern systems, and the significance of Calorific V
0 views • 27 slides
Thermochemistry
Thermochemistry is the study of the heat associated with chemical or physical changes, exploring concepts such as heat transfer, temperature, enthalpy, and specific heat capacity. This field delves into the relationships between heat, energy, and temperature changes in various substances.
0 views • 9 slides
Thermochemistry Fundamentals
Thermochemistry is the study of heat energy associated with chemical and physical changes. Learn about heat, temperature, enthalpy, calorimetry, specific heat capacity, and solve practice problems in your notebook.
1 views • 19 slides
Thermometric Titrations in Analytical Chemistry
Thermometric titrations, a method in analytical chemistry, involve continuous addition of a titrant to a reactant in an adiabatic vessel. The change in temperature due to the enthalpy change can be used to determine the titration endpoint. Redox reactions, being strongly exothermic, are ideal for th
0 views • 55 slides
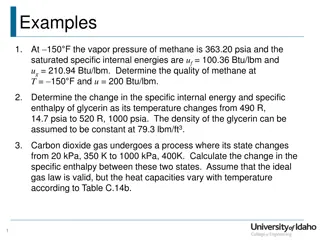
Determining Methane Quality and Specific Enthalpy Changes
Given the properties of methane at 150°F, determine the quality at a specific internal energy. Calculate the change in specific internal energy and specific enthalpy of glycerin as it undergoes a temperature change. Also, find the change in specific enthalpy for carbon dioxide gas in a varying temp
0 views • 5 slides
Understanding Flood Dynamics and Thermodynamic Calculations in Chemical Reactions
This content delves into the concepts of flooding, river flows, and thermodynamic calculations related to chemical reactions. It discusses the causes and impacts of floods, emphasizing how they occur in various river channels. Additionally, it explains the application of the first law of thermodynam
0 views • 9 slides
Exploring Statistical Thermodynamics in Physical Chemistry
Discover the fascinating world of statistical thermodynamics in physical chemistry, bridging the gap between macroscopic thermodynamics, kinetics, and microscopic atomic structures explained by quantum mechanics. Delve into topics such as the Boltzmann Distribution, partition functions, and the link
0 views • 26 slides
Heat Capacity, Enthalpy, Entropy, and Third Law of Thermodynamics
Explore the concepts of heat capacity, enthalpy, and entropy in the context of thermodynamics, including the molar values of properties, theoretical calculations of heat capacity, and the impact of temperature on energy levels. Learn about the Third Law of Thermodynamics and the relationships betwee
0 views • 24 slides
Understanding Enthalpies of Formation and Chemical Kinetics
Explore enthalpy diagrams, enthalpies of formation, and chemical kinetics in this comprehensive guide. Learn how the change in reaction enthalpies relates to enthalpies of formation and delve into the rates of reactions and chemical kinetics to understand the speed of chemical processes over time.
0 views • 38 slides
Understanding Biochemical Thermodynamics and Bioenergetics
Delve into the fascinating realm of biochemical thermodynamics and bioenergetics, which focus on energy changes in biochemical reactions such as exergonic and endergonic processes. Explore concepts like free energy, enthalpy, entropy, and their interrelations to predict the feasibility of chemical r
2 views • 8 slides
Understanding the Laws of Thermodynamics
Explore the fundamental laws of thermodynamics, starting with the first law that describes energy flow in closed systems through heat and work. Delve into scenarios involving internal energy changes, work calculations, and enthalpy considerations. Additionally, discover the implications of the secon
0 views • 22 slides
Chemistry Lecture on Thermodynamics and Gibbs Free Energy
Explore the concepts of enthalpy, entropy, and Gibbs free energy in chemistry, with a focus on thermodynamics and spontaneous processes. Learn about reaction heat, disorder of systems, and the spontaneity of processes based on Gibbs free energy calculations.
0 views • 20 slides
Thermodynamics Concepts and Perfect Gas Introduction at Universitas Nurtanio
Explore the fundamental concepts of thermodynamics, including entropy, engine cycles, and efficiency at Universitas Nurtanio. Learn about perfect gases and their properties, ideal gas state equation, internal energy, and enthalpy. Dive into examples and applications to deepen your understanding. Get
0 views • 57 slides