Hess's Law and Enthalpy Calculations
Learn how to apply Hess's Law to calculate enthalpy changes in chemical reactions. Discover the principles behind Hess's Law, its application in determining enthalpy changes, and verification methods through different routes. Explore diagrammatic representations and simultaneous equations illustrating the concept.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
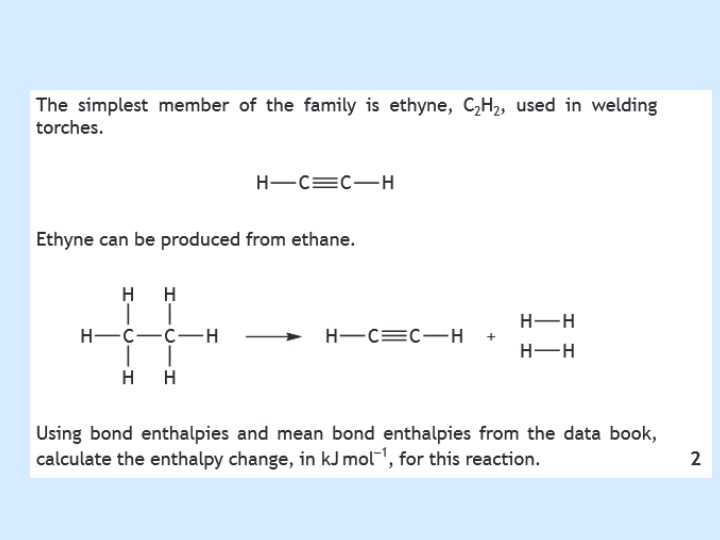
c) Hess s law Learning intention Learn how to calculate enthalpy changes for a application of Hess s Law. reaction by
Hesss Law and calculations Hess s law states that the enthalpy change for a reaction depends only on the initial and final states of the reaction and is independent of the route by which the reaction may occur.
Hess law can be shown diagrammatically in different ways e.g. carbon can burn to produce carbon dioxide directly by route X or it can form the intermediate CO by route Y, then the CO can burn to produce CO2 by route Z. C + O2 H = Y According to Hess law CO + O2 Enthalpy X = Y + Z H = X H = Z CO2
Hess law can be shown diagrammatically in different ways e.g. carbon can burn to produce carbon dioxide directly by route X or it can form the intermediate CO by route Y, then the CO can burn to produce CO2 by route Z. + O2 H = X C CO2 + O2 + O2 H = Z H = Y CO According to Hess law X = Y + Z
A more useful way is to set out the equations like simultaneous equations in maths. e.g. carbon can burn to produce carbon dioxide directly by route X or it can form the intermediate CO by route Y, then the CO can burn to produce CO2 by route Z. C + O2 CO2 H = X + O2 CO CO + O2 CO2 H = Y H = Z C CO2 H = X = Y + Z C + O2
Verification of Hesss Law H = enthalpy change Route 1 H 1 KOH (s) KCl (aq) H 2 + H2O(l) + HCl(aq) Route 2 H 3 KOH (aq) The conversion of solid KOH to KCl solution can be achieved by two possible routes. Route 1 is a single-step process, (adding HCl (aq) directly to the solid KOH) and Route 2 is a two-step process (dissolve the solid KOH in water, then adding the HCl(aq)) All steps are exothermic. If Hess s Law applies, the enthalpy change for route 1 must be the same as the overall change for route 2. H 1 = + H 2 H 3
Verification of Hesss Law Route 2 H 2 + H 3 Route 1 H 1 25 ml 1mol l-1 HCl 25 ml HCl 25 ml H2O then 1.2 g of KOH added to a polystyrene cup. Before adding the acid, its temperature is recorded. Slowly & continuously stir the reaction mixture with the thermometer until all the solid reacts. The highest final temperature after adding the acid is also recorded. 1. 1.2g of KOH added to a polystyrene cup. 2. Before adding the water, its temperature is recorded. The final temperature rise after adding the water is also recorded. Allow the solution to cool. 3. Record the temperature of the potassium hydroxide solution and the acid. 4. Now add the acid, again, recording the final temperature. Use the equation below to calculate H2 and H 3 H 1 = H 2 + H 3 will verify Hess s Law Knowing the specific heat capacity for water, it is then possible to calculate the Enthalpy change for this reaction. H 1 = cm T H = cm T
Given the equations Mg(s) + 2H+(aq) Mg2+(aq) + H2(g) H = a J mol 1 Zn(s) + 2H+(aq) Zn2+(aq) + H2(g) H = b J mol 1 Mg(s) + Zn2+(aq) Mg2+(aq) + Zn(s) H = c J mol 1 then, according to Hess s Law A c = a b B c = a + b C c = b a D c = b a.