Biochemical Reactions
Exploring the basics of chemical reactions, the conservation of matter principle, exothermic and endothermic reactions, and the role of activation energy in jumpstarting reactions. Learn how elements transform to create products, the significance of energy release or absorption, and the essential co
3 views • 19 slides
Aspen Simulation of Steam Reforming and Haber-Bosch Processes in Kinetics Reactors
Aspen simulation showcases the kinetics reactors for steam reforming of natural gas and the Haber-Bosch process for ammonia production. Steam reforming is highly endothermic, producing hydrogen and CO, while the Haber-Bosch process is exothermic, crucial for ammonia synthesis. The RPlug reactor and
4 views • 26 slides
Photosynthesis and Limiting Factors
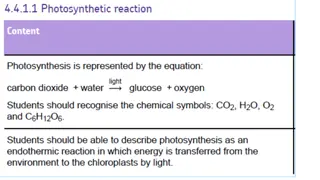
Photosynthesis is an endothermic reaction that takes in energy from its surroundings. The law of limiting factors explains how various factors such as light intensity, temperature, and CO2 concentration can impact the rate of photosynthesis. Additionally, the concept of the inverse square law helps
7 views • 45 slides
Energy Changes in Chemical Reactions
Exothermic reactions release energy to the surroundings, exhibited in processes like respiration and combustion. On the other hand, endothermic reactions absorb energy, demonstrated in examples such as photosynthesis. By observing changes in temperature and reactions between various substances, one
0 views • 24 slides
Changes of State in Matter
Explore the intriguing world of changes of state in matter, from melting to deposition. Learn about the various energy changes, particle behavior, and how substances transition between states by adding or removing energy like heat. Discover the differences between endothermic and exothermic changes
0 views • 25 slides
Chemical Reactions and Energy Transfers
Understanding chemical reactions involving thermal decomposition of metal carbonates, identifying exothermic and endothermic reactions based on energy transfers, and recognizing oxidation and reduction in redox reactions.
1 views • 5 slides
Phase Transformations and Latent Heat Equation in Statistical Mechanics
In this informative piece by Dr. N. Shanmugam, Assistant Professor at DGGA College for Women, Mayiladuthurai, the concept of phase transformations in substances as they change states with temperature variations is explored. The latent heat equation is discussed along with definitions of fusion, vapo
2 views • 22 slides
Energy Changes in Chemical Reactions Explained
Exothermic reactions release energy to the surroundings, examples include respiration and combustion, while endothermic reactions absorb energy, like in photosynthesis. By measuring temperature changes and observing reaction profiles, we can understand how bond-breaking and bond-making processes dri
0 views • 24 slides
Energy Changes in Chemical Reactions
Energy changes in chemical reactions can be categorized as exothermic and endothermic. Exothermic reactions release energy to the surroundings, while endothermic reactions absorb energy from the surroundings. Examples and uses of both types of reactions are provided, along with details on measuring
4 views • 24 slides
Temperature Control Strategies in Industrial Reactor Design
Temperature control plays a critical role in reactor design, especially when dealing with highly exothermic or endothermic reactions. Adiabatic operation is typically the preferred choice, but various methods such as heat-exchanger reactors, diluent usage, and inter-cooler systems are employed based
0 views • 20 slides
Chemical Bonding: Valency, Formulas, and Reactions
Explore the world of chemical bonding with this unit covering valencies, chemical formulas, ionic vs. covalent bonds, and exothermic vs. endothermic reactions. Learn to predict element combinations, create molecular formulas, and differentiate between various bond types. Jigsaw diagrams demonstrate
1 views • 46 slides
Thermochemistry: Key Concepts and Examples
Energy is fundamental in thermochemistry, where we explore its conversion forms, such as potential and kinetic energy. State functions and chemical energy play crucial roles in exothermic and endothermic reactions, affecting stability and bonds. Dive into the world of energy transformations with pra
0 views • 49 slides
Chemical Substances: Dissolved in Water
This article delves into the properties of various chemical substances when dissolved in water, including Calcium Carbonate, Sodium Carbonate, Iron Oxide, Magnesium Sulfate, and Potassium Chloride. It discusses the exothermic and endothermic reactions that occur during dissolution, along with the ch
0 views • 6 slides
Chemical Kinetics and Equilibrium in Reactions
Explore the basic concepts of reaction rates, collision theory, activation energy, and energy diagrams in chemical kinetics and equilibrium. Learn how particles must collide with the correct orientation and enough energy to form an unstable activated complex. Discover the role of activation energy i
0 views • 24 slides
Chemical Reactions and Catalysts
Chemical reactions involve the formation of new substances from reactants, with key processes like oxidation and reduction. Reversible reactions, endothermic and exothermic reactions, and the role of catalysts in speeding up reactions are explored. The significance of chemical symbols, formulas, and
0 views • 8 slides
Therm: Exploring Heat and Temperature Concepts
Explore the world of therm with a focus on heat and temperature-related terms like thermophile, exothermic, and endothermic. Learn about the unit of heat, calories, and how heat is related to energy forms. Discover the significance of absorbing heat and the organisms adapted to high temperature envi
0 views • 21 slides
Basics of Thermodynamics and Energy Principles
Explores fundamental concepts in thermodynamics, including energy, the first law of thermodynamics, heat vs. temperature, system vs. surroundings, direction of heat flow, thermal equilibrium, exothermic vs. endothermic processes, and units of energy. Learn about energy conversions, conservation, and
0 views • 24 slides
Chemical Reactions and Enzymes
Chemical reactions involve the transformation of chemicals by breaking and forming chemical bonds. Energy is released or absorbed during these processes. Reactions that release energy are exothermic, while those that absorb energy are endothermic. Catalysts help speed up reactions by lowering the ac
0 views • 13 slides
Chemical Kinetics: Reaction Rates and Activation Energy
Exploring the fundamental concepts of chemical kinetics, this content delves into reaction rates, collision theory, and activation energy in chemical reactions. It emphasizes the importance of particle collisions, correct orientation, and energy requirements for reactions to occur. Through energy di
0 views • 17 slides
Endothermic and Exothermic Reactions in Chemistry
Exploring endothermic and exothermic reactions, this activity involves measurements to determine heat release or absorption. Students create hypotheses and test them using Labidisc temperature sensors. Theoretical background on chemical reactions, energy absorption, and heat release is provided, ena
0 views • 20 slides
Exothermic and Endothermic Reactions of Different Substances
Learn about the concepts of exothermic and endothermic reactions, featuring specific substances like sodium hydroxide, lithium nitrate, ammonium nitrate, crystalline ammonium nitrate, and potassium bromide. Discover their properties, uses, and how they interact with water. Explore how these reaction
0 views • 5 slides
Exothermic and Endothermic Reactions in Chemistry
In chemistry, it's essential to differentiate between exothermic and endothermic reactions based on the direction of heat flow. Exothermic reactions release heat to the surroundings, while endothermic reactions absorb heat to proceed. Through examples like the combustion of propane and the formation
0 views • 21 slides
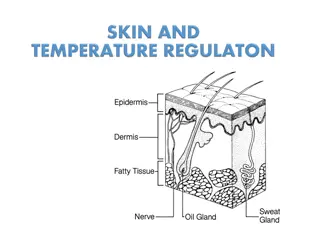
Thermoregulation in Animals and Humans
Thermoregulation is the process of regulating body temperature in organisms. It can be seen in two types of animals - ectothermic and endothermic. Ectothermic animals rely on external energy sources to maintain body temperature, while endothermic animals use internal metabolic energy. The skin plays
0 views • 22 slides
Endothermic and Exothermic Reactions Through Experiment
Explore the concepts of endothermic and exothermic reactions with a detailed experiment to determine which solute dissolves most endothermically and exothermically in water. Discover the roles of heat transfer in chemical reactions using potassium chloride, calcium chloride, sodium carbonate, and so
0 views • 11 slides
Heat Transfer in Phase Changes of Water
Water molecules exhibit different behaviors in the liquid and gaseous states due to varying attractions between molecules. To change liquid water to a gas, energy must be added to overcome intermolecular forces, making this process endothermic. The heat absorbed during melting is equal to the heat r
1 views • 22 slides
Chemical Reactions: Testing Substances Dissolved in Water
Explore the fascinating world of chemical reactions by testing various substances dissolved in water. Discover how salt, sugar, baking soda, borax, and vinegar interact with water, leading to endothermic or exothermic reactions with unique properties and uses.
0 views • 6 slides
Testing Chemical Substances Dissolved in Water
Explore the effects of salt, sugar, baking soda, borax, and vinegar when dissolved in water. Discover their properties, reactions, and practical applications, shedding light on endothermic and exothermic reactions in aqueous solutions. Delve into the chemical compositions and behaviors of these comm
0 views • 6 slides
Investigating Chicken Liver Reactivity with Hydrogen Peroxide Using Peroxidase Lab
Explore the reaction between chicken liver and hydrogen peroxide in this peroxidase lab experiment. Learn about peroxidase enzymes that break down hydrogen peroxide into less harmful substances. Follow the procedure using materials like an Erlenmeyer flask, toothpick, and candle to observe reactions
0 views • 11 slides
CHEMISTRY INTERDISCIPLINARY PROJECT
Endothermic and exothermic reactions involve heat energy absorption or release during chemical processes. Examples of substances exhibiting these behaviors include Ammonium chloride and Potassium nitrate as endothermic, and Sodium hydroxide as exothermic. Explore their properties, applications, and
0 views • 11 slides