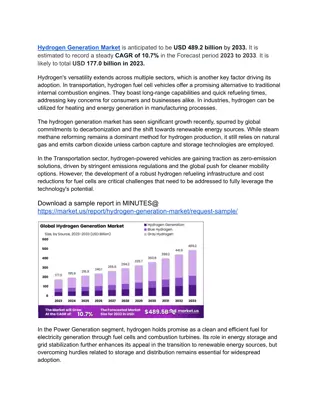
Investigating Chicken Liver Reactivity with Hydrogen Peroxide Using Peroxidase Lab
Explore the reaction between chicken liver and hydrogen peroxide in this peroxidase lab experiment. Learn about peroxidase enzymes that break down hydrogen peroxide into less harmful substances. Follow the procedure using materials like an Erlenmeyer flask, toothpick, and candle to observe reactions and record findings. Formulate a hypothesis, record observations, and classify reactions as endothermic or exothermic based on clues observed.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. Download presentation by click this link. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
E N D
Presentation Transcript
What is peroxidase? All organisms have enzymes (catalysts) called peroxidases that break down hydrogen peroxide into less harmful substance. Hydrogen Peroxide (H2O2) is a pale blue liquid that appears clear when diluted. Hydrogen peroxide is a weak acid that is used as a bleaching agent and disinfectant.
Do Learn Connect Do: Peroxidase Lab Learn: I can identify the products of a reaction and classify reactions in terms of energy. Connect: Properties and Patterns, Energy
Question: Will chicken liver react with hydrogen peroxide and if so what products are formed?
Hypothesis If chicken liver reacts with hydrogen peroxide, then I will observe __________ (chemical reaction clues) and ____________ will be formed (products) because ______________________________ ______________________________ ______________________________ ______
Materials Chicken Liver Hydrogen Peroxide Erlenmeyer Flask Stopper Toothpick Candle
Procedure Add Hydrogen Peroxide to flask. Stopper the flask. Record observations Remove stopper from flask and insert toothpick into neck of flask. Do not drop it. Record observations Stopper flask. Use the candle to ignite the toothpick, blow out the flame. Remove stopper from flask and insert toothpick into neck of flask. Do not drop it.
Procedure Add a small amount of chicken liver to the flask. Stopper the flask. Record observations. After about 2 minutes, carefully feel the bottom of the flask. Record Observations Ignite the toothpick and blow out the flame. Remove stopper and insert toothpick into the neck of the flask. Do not drop it. Record observations. Stopper the flask and repeat the last steps three more times.
Consider these questions: Why did you make observations after each step? What observations indicated that a chemical change took place? (Clues) Classify the reaction as endothermic or exothermic using evidence. What products were formed in this reaction? Why is it important to replace the stopper quickly when it had been removed?
Products The equation for the reaction is : 2H2O2 ________ and ________
CER (Conclusion) Claim: The chicken liver did react with the hydrogen peroxide forming ______________. (Products) Evidence: During the chemical reaction, I observed ________________. (Clues) Reasoning: Provide your own reasoning connecting your products and clues to chemical reactions. Pre-Ap should also connect to ENERGY.