Canizzaro Reaction in Organic Chemistry: Experiment and Applications
The Canizzaro reaction involves the disproportionation of aldehydes in the presence of a strong base to produce an alcohol and a carboxylic acid. This experiment, supervised by Lecturer Israa Radhi, explores the mechanism and practical application of the reaction. Benzyl alcohol and benzoic acid, pr
1 views • 7 slides
Adhesives which harden without chemical reaction
Adhesives can harden without a chemical reaction, using water-soluble materials, or through the loss of solvents. Environmental pressures are leading to the development of water-based adhesives to replace solvent-based ones. Various adhesives like neoprene adhesives and starch-based pastes offer dif
1 views • 17 slides
Understanding the Diels-Alder Reaction in Practical Organic Chemistry
The Diels-Alder reaction is a fundamental method in organic chemistry for producing cyclic organic compounds by combining a conjugated diene with an alkene. This reaction, named after Otto Diels and Kurt Alder, involves the formation of a six-membered ring with specific bond rearrangements. Conjugat
4 views • 15 slides
Chemical Kinetics: Understanding Reaction Rates and Factors
Chemical kinetics is a branch of physical chemistry that explores the velocity and factors influencing chemical reactions. It studies how reactants transform into products, considering conditions like temperature, pressure, and reactant concentrations. Factors affecting reaction rates include the na
7 views • 24 slides
Cannizzaro Reaction
The Cannizzaro reaction is a chemical reaction involving the base-induced disproportionation of non-enolizable aldehydes to form a primary alcohol and a carboxylic acid. Discover more about this reaction, its history, mechanism, and variants like the Cross Cannizzaro reaction and Intramolecular Cann
1 views • 20 slides
Benzoin Condensation: A Name Reaction Explained by Dr. Atul Kumar Singh
Benzoin condensation is a classic organic reaction where aromatic aldehydes self-condense to form α-hydroxy ketones. Dr. Atul Kumar Singh, an Assistant Professor of Chemistry, details the mechanism and the specific catalytic properties of cyanide in this reaction. The reaction involves refluxing th
0 views • 6 slides
Investigating Impact of Practice on Human Reaction Time Through Ruler Drop Test
This practical investigation focuses on determining if practice can reduce human reaction times by conducting a ruler drop test. Participants use their weaker hand to catch a ruler dropped by their partner, aiming to improve their reaction time with practice. The experiment explores how athletes can
0 views • 7 slides
Understanding Chemical Kinetics: Rates of Reactions and Factors Influencing Them
Chemical kinetics delves into the speed of chemical reactions and the factors that influence reaction rates. This field explores how collisions between atoms, ions, or molecules drive chemical reactions, as well as the role of catalysts, reactant concentration, temperature, and surface area. By unde
0 views • 32 slides
Kinetic Reaction of Sulphite and Iodate - Landolt Reaction Overview
The kinetic reaction of sulphite ions and iodate in the Landolt reaction is a fascinating chemical process where slow and fast reactions occur sequentially, resulting in a visually striking color change. By monitoring the induction period between the two reactions, one can observe the formation of h
0 views • 9 slides
Understanding Chemical Kinetics: Rates, Reactions, and Mechanisms
Chemical kinetics involves studying reaction rates, rate laws, stoichiometry, and factors affecting reaction speed. This branch of chemistry delves into determining reaction orders, rate constants, and activation energies using various methods. Different types of rates, such as initial, instantaneou
2 views • 68 slides
Understanding the Prins-Pinacol Reaction in Organic Chemistry
The Prins-Pinacol reaction involves a two-step process starting with the Prins reaction and followed by the Pinacol rearrangement. This reaction, discovered in 1919 by Hendrick J. Prins, is a crucial transformation in organic chemistry, leading to the formation of important carbonyl compounds. The m
0 views • 14 slides
Understanding Kinetics and Reaction Rates in Chemistry
Kinetics is the study of reaction rates and factors affecting them, such as concentration, temperature, catalysts, and more. Orders of reaction classify reactions based on rate dependency on reactant concentration. Factors like pH, light, and solvents can also impact reaction rates. Half-life and sh
0 views • 18 slides
Understanding Chemical Kinetics: Reaction Rates and Mechanisms
Chemical kinetics is a branch of chemistry focused on studying reaction rates and mechanisms. Unlike thermodynamics, which deals with feasibility, kinetics explores the speed at which reactions occur. Factors such as temperature, pressure, and catalysts influence reaction rates. Understanding the ra
3 views • 72 slides
Understanding Reaction Rates in Chemistry: Apparatus, Experiment, and Analysis
Explore the concept of reaction rates in chemistry through the use of a gas syringe apparatus, conducting experiments, analyzing results, and understanding factors affecting reaction rates. Dive into hands-on activities and graphical representations to enhance your understanding of this fundamental
0 views • 16 slides
Exploring Reaction-Diffusion Systems and Random Walks in Chemistry
Delve into the fascinating world of reaction-diffusion systems and random walks in chemistry, exploring concepts such as well-mixed reactive systems, diffusion-reaction dynamics, finite differences, and incorporating reactions into random walks. Discover how these principles play a crucial role in u
0 views • 29 slides
Understanding Flow Chemistry for Efficient Chemical Reactions
Flow chemistry, also known as continuous flow or plug flow chemistry, revolutionizes chemical reactions by running them in a continuous flow stream. This dynamic process offers efficient manufacturing of chemical products with precise control over critical parameters like stoichiometry, mixing, temp
2 views • 7 slides
Understanding Stoichiometry in Chemical Reactions
Stoichiometry is the concept of predicting the amounts of reactants and products in a chemical reaction, similar to following a recipe in cooking. It involves balancing chemical equations and determining the quantities of substances involved. By paying attention to coefficients, one can calculate ho
2 views • 65 slides
Utilizing a Global Model for Analyzing Reaction Pathways in Plasma Systems
This research focuses on using a kinetic global model framework to identify relevant reactions in chemically complex plasma systems. The framework, KGMf, enables the investigation of macroscopic plasma characteristics by analyzing reaction pathways, sensitivity to reaction rate errors, and dominant
1 views • 6 slides
Fundamentals of Chemical Kinetics and Reactor Design
Explore the realm of chemical reactions, rate equations, and reactor design in this informative chapter. Understand the factors influencing reaction rates, different types of reactions, rate laws, and experimental determination of reaction rates. Dive into examples illustrating stoichiometry and rat
0 views • 19 slides
Understanding Chemical Reaction Kinetics in Chemcad
Explore the kinetic reactor module in Chemcad for reaction rate specification using VBA. Learn how to determine parameter values and analyze reactions such as steam reforming and water gas shift. Follow step-by-step instructions to run the unit operation, view reactor profiles, and compare reaction
0 views • 9 slides
Chemical Reactor Design: Rates, Mechanisms, and Reactor Types
The field of chemical reactor design encompasses studying reaction rates, mechanisms, and designing reactors for various processes. Factors such as reaction type, production scale, cost, safety, and more influence the choice of reactor scheme. Understanding reactor systems and applying engineering j
1 views • 15 slides
Overview of Chemical Reactor Design and Operation
Chemical reactor design involves studying the rates and mechanisms of chemical reactions, as well as the design of reactors for these reactions on a commercial scale. This field combines principles from thermodynamics, chemical kinetics, fluid mechanics, mass transfer, heat transfer, and economics t
0 views • 12 slides
Calculating Limiting Reagent in Chemical Reactions
Calculating the amount of reactants in excess and the limiting reagent plays a crucial role in determining the maximum extent of a chemical reaction. By using the relative numbers of moles of substances as shown in balanced equations, one can identify the reactant that is fully utilized, hence limit
0 views • 14 slides
Understanding Chemical Reaction Kinetics: From Unimolecular to Three-Body Reactions
Explore the fundamental concepts of chemical reactions, including unimolecular reactions like thermolysis and photolysis, bimolecular reactions, and three-body reactions. Learn about rate constants, reaction mechanisms, and the impact of pressure on reaction rates. Discover how energy transfer, phot
0 views • 9 slides
Understanding Free Energy, Reaction Quotient, and Equilibrium Constant
This educational material delves into the concepts of free energy, reaction quotients, and equilibrium constants in chemical systems. It explains how to determine the direction of a reaction based on Q and K values, elucidates the role of Gibbs free energy in determining spontaneity, and provides ca
0 views • 10 slides
Understanding Chemical Equilibrium in Reversible Reactions
Chemical equilibrium occurs when the concentrations of reactants and products remain constant over time in a reversible reaction. Reaction rate is proportional to concentration, and equilibrium is reached when the rate of formation equals the rate of consumption in both directions. Reversible reacti
0 views • 25 slides
Systems-Oriented Concept Map Extension for Reactive Nitrogen Flows
International Organization for Chemical Sciences in Development presents a Systems-Oriented Concept Map Extension (SOCME) focusing on biogeochemical flows of reactive nitrogen from NH3. The concept explores core reaction subsystems, energy input subsystems, equilibrium conditions, and the integratio
0 views • 11 slides
Understanding Chemical Reaction Factors
Explore the impact of temperature, concentration, catalysts, and surface area on the rate of chemical reactions. Learn how these factors influence reaction speed through real-life applications and experiments such as Alka-Seltzer in water and magnesium ribbon with different HCl concentrations.
0 views • 19 slides
Grignard Reaction in Chemistry Lab: Part 1 Overview
The Grignard Reaction Part 1 in Chemistry 318 Fall 2018 involves the preparation of the Grignard reagent, its reaction with CO2, and the isolation of the benzoic acid product. The experiment spans two lab sessions, focusing on safety precautions, pre-lab checks, and upcoming due dates. Students are
0 views • 11 slides
Introduction to Chemical Reaction Engineering (CRE)
Chemical Reaction Engineering (CRE) focuses on studying the rates and mechanisms of chemical reactions, as well as designing reactors for these reactions. The field involves understanding balances in terms of molar flow rates, mole balances, rate laws, stoichiometry, and membrane reactors. Membrane
0 views • 20 slides
Understanding Chemical Kinetics: The Rate of Reaction and Equilibria
Chemical kinetics explores the rate at which chemical reactions occur and the factors influencing them. This tutorial delves into the concepts of reaction rates, equilibrium, collision theory, and the role of concentration in determining reaction rates. By understanding these principles, industries
0 views • 117 slides
Understanding Rates of Chemical Reactions: Factors and Effects
Rates of chemical reactions are determined by the Collision Theory, which explains how reaction rates are influenced by factors such as concentration, surface area, temperature, catalysts, and stirring. Increasing concentrations, surface area, and temperature generally lead to faster reaction rates,
1 views • 8 slides
Understanding Chemical Reactions in Daily Life
Understanding chemistry, particularly chemical reactions, is crucial for our daily lives. Chemical reactions involve the transformation of substances into different ones, described by reactants and products in equations. By learning about chemical equations, word equations, formula equations, and th
0 views • 15 slides
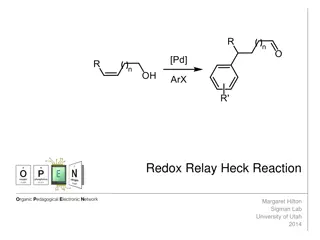
Understanding the Redox-Relay Heck Reaction in Organic Synthesis
The Redox-Relay Heck Reaction is a powerful tool in organic synthesis that allows for the functionalization of olefins with aryl groups. Developed by Sigman and colleagues, this reaction involves a palladium-catalyzed relay controlled by a thermodynamic sink, leading to the formation of aldehydes or
0 views • 6 slides
Understanding Chemical Kinetics: Reaction Rates and Activation Energy
Exploring the fundamental concepts of chemical kinetics, this content delves into reaction rates, collision theory, and activation energy in chemical reactions. It emphasizes the importance of particle collisions, correct orientation, and energy requirements for reactions to occur. Through energy di
0 views • 17 slides
Understanding Volumetric Analysis in Chemical Experiments
Chemical analysis is crucial in studying material composition. Volumetric analysis, a key procedure, involves measuring reaction volumes in solutions to determine substance concentrations. This method utilizes titration and different chemical reaction types like acid-base and precipitation methods.
0 views • 17 slides
Understanding Homogenous Chemical Equilibrium
Homogenous chemical equilibrium occurs when reactants and products are in the same phase. This equilibrium remains independent of the volume of the reaction mixture. The concept is illustrated through the example of the Hydrogen-Iodide system and a generic reaction A + B --> 2C. Partial pressure pla
0 views • 56 slides
Chemical Reactions: Writing, Balancing, and Mastering Skills
Explore the essential topics of balancing chemical reactions, determining reaction occurrence, memorization tips, and using solubility tables. Master the art of writing and balancing reactions through fundamental rules, techniques, and practice examples. Understand the basics of chemical reactions,
0 views • 20 slides
Introduction to Chemical Reaction Engineering
Chemical Reaction Engineering (CRE) is crucial for understanding how chemical reactors operate in various processing operations. This field involves reactor design by integrating factors such as thermodynamics, kinetics, fluid mechanics, heat transfer, and economics. CRE aims to effectively design a
0 views • 16 slides
Understanding Kinetics in Chemical Reactions
Kinetics is the study of reaction rates and factors affecting them. Reaction rate is the speed at which a reaction occurs, influenced by factors like concentration, temperature, pH, light, catalysts, and solvents. Reactant concentration determines reaction order, which categorizes reactions as zero-
0 views • 18 slides