Organometallic Chemistry (CHEM 42 1)
Organometallic chemistry delves into compounds with carbon-metal bonds, merging concepts from inorganic and organic chemistry. The field encompasses diverse compounds like ferrocene and tris(triphenylphosphine)rhodium carbonyl hydride, with nomenclature based on naming organic groups and adding meta
1 views • 13 slides
Aldol Condensation Reaction: Preparation of Chalcones
Chalcones are important unsaturated aromatic ketones that serve as biogenetic precursors of flavonoids and isoflavonoids. They have various medicinal and pharmaceutical applications due to their biological activities. Chalcones are easily synthesized compounds with potential therapeutic uses, making
2 views • 13 slides
Transition Metals Organometallic Compounds Overview
Transition metals bonded with organometallic compounds like metal alkyls, aryls, and hydrides are common in chemistry. Ligands are crucial for stabilizing these complexes, with carbon-based ligands exhibiting diverse binding modes based on the metal's hybridization state. Preparation methods for tra
1 views • 25 slides
Crystal Field Theory in Transition Metal Complexes
Crystal Field Theory (CFT) explains the colors and magnetic properties of transition metal complexes. It focuses on the energy changes in d-orbitals of metal ions caused by surrounding ligands. This theory, developed in 1929, provides insights into the bonding interactions in complex compounds. The
10 views • 44 slides
Pharmaceutical Chemistry Analgesic Agents Narcotic Analgesics
Analgesic agents play a crucial role in managing pain, ranging from mild to severe, with different categories such as opioids, NSAIDs, and triptans. The origin of pain can vary from physiological to neuropathic causes. Opioids target opioid receptors, with discoveries in endogenous ligands like enke
4 views • 49 slides
Ligands and Metal Carbonyl Complexes: A Comprehensive Overview
Explore the classification of ligands in metal complexes, including alkyl, aryl, and carbonyl ligands. Learn about the unique properties of carbonyl ligands, their preparation methods, and the molecular orbital diagram illustrating CO-metal bonding interactions.
5 views • 35 slides
Understanding Osazone Test: A Chemical Test for Detecting Reducing Sugars
The Osazone test is a chemical test used to detect reducing sugars by forming derivatives of carbohydrates with phenyl hydrazine. This test helps in distinguishing different reducing sugars and differentiating between reducing and non-reducing sugars. The principle involves the reaction between carb
11 views • 22 slides
Phenolic Resin
Phenolic resins, the oldest synthetic polymer, are produced by reacting phenols with aldehydes, primarily phenol and formaldehyde. This process was invented in 1907 and has since been crucial in manufacturing durable molded parts. Phenols, aromatic compounds with unique properties, play a key role i
1 views • 24 slides
Organometallic Chemistry III: Transition Metal Complexes and Homogeneous Catalysis
Explore the reactivity of transition metal complexes, including bond metatheses and various reactions. Learn about orbital considerations, synthesis, and spectroscopic properties of organometallic complexes. The course covers basics from AC1, focusing on ligands, electron counting, and MO diagrams.
5 views • 8 slides
Understanding Narcotic Analgesics and Opiates: History, Mechanisms, and Uses
Delve into the world of narcotic analgesics and opiates, exploring the history of opium poppy, morphine derivatives, opioid compounds, and the pharmacology mechanisms of action. Discover the uses of opiates in analgesia, preanesthetic medication, and more, alongside the endogenous ligands involved.
8 views • 55 slides
Understanding Crystal Field Theory in Chemistry
Crystal Field Theory (CFT) explains how electron orbital degeneracies, particularly d or f orbitals, are affected by a static electric field generated by neighboring anions. In CFT, the metal ion is considered positive while ligands are negative charges, leading to attractive and repulsive forces af
0 views • 13 slides
Understanding Hard and Soft Acids and Bases (HSAB Principles) by Dr. Gurpreet Kaur
Delve into the world of Hard and Soft Acids and Bases (HSAB) with Dr. Gurpreet Kaur as she explains the characteristics of hard and soft acids, Pearson's HSAB principle, applications such as predictions of coordination in complexes, poisonings of metal catalysts, and the classification of acids and
2 views • 17 slides
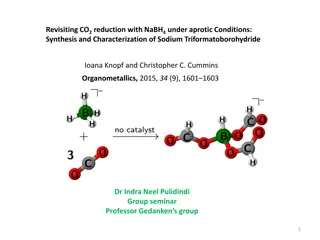
Sodium Triformatoborohydride Formation via NaBH4-CO2 Reaction
Sodium triformatoborohydride, Na[HB(OCHO)3], is synthesized by reacting NaBH4 with CO2 under aprotic conditions. The choice of reductant is crucial for CO2 reduction methods, highlighting the role of NaBH4 as a cost-effective and reactive reducing agent for formate production. The exclusive formatio
1 views • 12 slides
Dakin Rearrangement in Organic Chemistry: Mechanism and Positional Effects
The Dakin Rearrangement, also known as Dakin oxidation, is an organic redox reaction involving hydroxylated phenyl aldehydes or ketones reacting with hydrogen peroxide to form benzenediols and carboxylates. The mechanism includes nucleophilic addition, [1,2]-aryl migration, and final product formati
1 views • 9 slides
Understanding Coordination Compounds and Ligands in Chemistry
Coordination compounds involve ligands that donate electron pairs to central metal ions. Ligands can be categorized based on the number of donor atoms they contain, such as mono-, bi-, tri-, tetra-, penta-, and hexadentate ligands. Each type of ligand has the ability to form bonds with the central m
2 views • 15 slides
Understanding Complex Ions and Coordinate Bonds in Chemistry
Complex ions in chemistry are formed when transition metals or their ions bond with ligands through coordinate bonds. Ligands utilize their lone pairs of electrons to form dative covalent bonds with transition metals, determining the coordination number of the cation. Complex ions play a crucial rol
1 views • 29 slides
Understanding Enemark-Feltham Notation in Iron-Nitrosyl Complexes
Iron-Nitrosyl complexes are redox non-innocent, with NO exhibiting multiple redox states. Enemark-Feltham Notation helps in determining metal-ligand interactions and oxidation states. Detailed information on NO ligands, bonding characteristics, and methods for analyzing iron-NO systems are discussed
0 views • 6 slides
Understanding the Prins-Pinacol Reaction in Organic Chemistry
The Prins-Pinacol reaction involves a two-step process starting with the Prins reaction and followed by the Pinacol rearrangement. This reaction, discovered in 1919 by Hendrick J. Prins, is a crucial transformation in organic chemistry, leading to the formation of important carbonyl compounds. The m
0 views • 14 slides
Understanding Chemical Groups and Macromolecules in Biological Processes
In biological processes, certain chemical groups play crucial roles in molecular functions. These functional groups, including hydroxyl, carbonyl, carboxyl, amino, sulfhydryl, phosphate, and methyl, are essential for the structure and function of biological molecules. Additionally, macromolecules, s
0 views • 9 slides
Organic Chemistry Fundamentals: Aldehydes and Ketones
The chapter explores aldehydes and ketones, common classes of carbonyl compounds, the structure of the carbonyl group, and the nomenclature of aldehydes according to the IUPAC system. It discusses the characteristics, bonding, and nomenclature rules associated with these important functional groups
0 views • 22 slides
Understanding Aldehydes and Ketones in Organic Chemistry
This chapter discusses carbonyl compounds focusing on aldehydes and ketones. It covers their structural differences, drawing, nomenclature, physical properties, synthesis methods, and nucleophilic attack reactions. Additionally, it includes common classes of carbonyl compounds, naming conventions fo
0 views • 21 slides
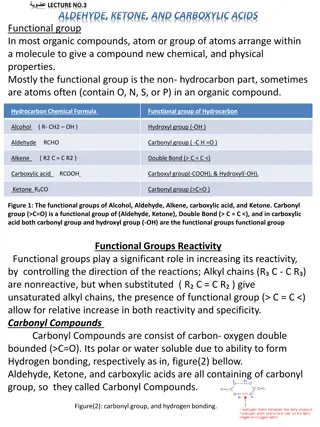
Understanding Aldehydes, Ketones, and Carboxylic Acids in Organic Chemistry
Functional groups like aldehyde, ketone, and carboxylic acids play a crucial role in organic compounds, influencing reactivity and properties. Carbonyl compounds containing carbon-oxygen double bonds exhibit unique characteristics. Ketone bodies have biological significance, especially in conditions
0 views • 12 slides
Geometrical Isomerism in Octahedral Complexes: A Comprehensive Overview
Geometrical isomerism in octahedral complexes is a fascinating phenomenon arising from different geometric arrangements of ligands. This type of isomerism is prevalent in coordination numbers 4 and 6, leading to two main types of geometric isomers. Examples of cis-trans and mer-fac isomers in MA2B4
5 views • 10 slides
Understanding Carbohydrates: Essential Organic Molecules in Nature
Carbohydrates, also known as saccharides, are crucial organic compounds that serve various functions in living organisms. They are a major energy source, play a role in energy storage, contribute to cell structure, and are essential components of DNA and RNA. Carbohydrates are classified into four g
0 views • 80 slides
Understanding Aldehydes and Ketones in Organic Chemistry
Aldehydes and ketones are compounds that contain carbonyl groups (>C=O). Aldehydes have the CO group linked to either two hydrogen atoms or one hydrogen atom and one alkyl or aryl group, while ketones have the CO group linked to two alkyl or aryl groups. The structure of the carbonyl group is charac
0 views • 26 slides
Baeyer-Villiger Oxidation: Mechanism and Migration Aptitude
The Baeyer-Villiger oxidation is a key organic reaction that transforms ketones into esters or cyclic ketones into lactones using peroxyacids. The mechanism involves protonation of the carbonyl group and migration of substituents. Migration aptitude in this reaction determines the regioselectivity,
0 views • 10 slides
Schiff bases
Schiff bases are condensation products of primary amines and carbonyl compounds, discovered by German chemist Hugo Schiff in 1864. They play vital roles in enzymatic reactions, coordination chemistry, and are used in diverse biological aspects due to their antibiotic, antiviral, and antitumor proper
0 views • 10 slides
Role of Adhesion Molecules in Immune Response to SARS-CoV-2 Engagement
Engagement of SARS-CoV-2 triggers the complement system and TLR 7 in neutrophils and macrophages, leading to the release of inflammatory cytokines and chemotactic factors. This results in the upregulation of adhesion molecules on blood capillary endothelial cells and leukocytes, facilitating leukocy
3 views • 12 slides
Affinity Chromatography: A Breakthrough in Biochemical Research
Affinity chromatography, developed in the 1930s by A. Wilhelm Tiselius, is a vital technique for studying enzymes and proteins. It relies on the specific affinity between biochemical compounds and utilizes matrices like agarose for binding sites. Ligands such as amino and hydroxyl groups play crucia
1 views • 27 slides
Exploring Bioinorganic Chemistry: Connecting Inorganic and Biochemistry
Bioinorganic Chemistry bridges the gap between inorganic chemistry and biochemistry, understanding the vital role of inorganic elements in living systems. This interdisciplinary field delves into the structure, function, and exploitation of metal ions in biological processes, emphasizing their inter
0 views • 47 slides
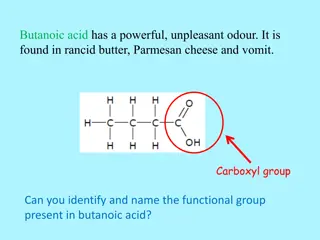
Understanding Functional Groups in Organic Molecules
Discover the key functional groups present in various organic compounds such as butanoic acid, methanal, Dettol, 4-formamidobenzoic acid, cinnamon, methyl anthranilate, and vitamin C. Learn to identify and name these functional groups, including carboxyl, carbonyl, hydroxyl, amide, ester link, and a
0 views • 12 slides
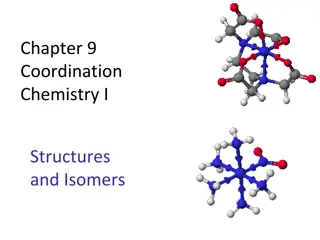
Understanding Coordination Chemistry: Structures, Isomers, and Naming
Exploring coordination chemistry involves understanding structures, isomers, naming conventions, and common coordination numbers, all essential in studying coordination compounds. Coordination compounds consist of central metals, ligands, and charge balancing ions. Naming involves listing cations, l
0 views • 46 slides
Insights into Coordination Chemistry Elements and Complexes
Transition elements with d or f electrons possess unique properties, play crucial roles in biological processes, and form colorful complexes with ligands. Occurring widely in nature, these elements have varied oxidation states and coordination numbers. Werner's formulation sheds light on primary and
0 views • 41 slides
Understanding Organic Chemistry: Functional Groups and Naming Rules
Delve into the world of organic chemistry with a focus on functional groups like alcohols, ethers, aldehydes, and ketones. Explore the rules for naming these compounds, understanding their structures, and how they impact the properties of molecules. From alcohols with hydroxy groups to ketones conta
0 views • 21 slides
Understanding the Pinacol-Pinacolone Rearrangement in Organic Chemistry
The Pinacol-Pinacolone rearrangement is a crucial process in organic chemistry for converting 1,2-diols into carbonyl compounds. This rearrangement involves a 1,2-migration under acyl conditions, resulting in the shift of two adjacent atoms. Learn about the mechanisms, products, and uses of Pinacolo
0 views • 11 slides
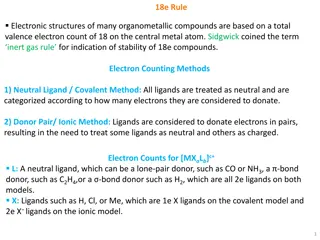
Understanding the 18e Rule in Organometallic Compounds
The 18e rule dictates the electronic structures of many organometallic compounds, emphasizing a total valence electron count of 18 on the central metal atom for stability. Electron counting methods like the Covalent and Ionic models assist in determining the electron distribution among ligands. The
0 views • 8 slides
Understanding Ultraviolet and Visible Spectroscopy in Chemistry
Ultraviolet and visible spectroscopy in chemistry involves the absorption of light energy by molecules, dependent on their electronic structure. This process, also known as electronic spectrum, entails energy transitions of electrons in molecular orbitals. The region of the electronic spectrum inclu
0 views • 29 slides
Understanding Coordination Complexes and Transition Metals
Today's lecture covers transition elements, coordination complexes, ligand types, geometries, naming, isomers, and bonding in coordination complexes. Transition metals form coordination complexes with metal ions, ligands, and counter ions. The types of ligands include monodentate and bidentate ligan
0 views • 24 slides
Understanding Chelation Chemistry: Structural Requirements, Ligands, and Applications
Chelation chemistry involves the formation of specific complexes known as chelates, characterized by ligands that coordinate with a central metal ion. This article explores the structural requirements for chelate formation, the role of chelating agents like EDTA and DMG, and the difference between m
0 views • 27 slides
Exploring Ligands for Nanoparticle Surface Effects
Design and Synthesis of Ligands for studying Nanoparticle Surface Charge and Distribution, including examples of monomeric and polymeric ligands. Critical design elements of the ligand library are discussed, emphasizing scalable synthesis and key features. Initial synthesis challenges are noted, wit
0 views • 14 slides