Sodium Triformatoborohydride Formation via NaBH4-CO2 Reaction
Sodium triformatoborohydride, Na[HB(OCHO)3], is synthesized by reacting NaBH4 with CO2 under aprotic conditions. The choice of reductant is crucial for CO2 reduction methods, highlighting the role of NaBH4 as a cost-effective and reactive reducing agent for formate production. The exclusive formation of Na[HB(OCHO)3] is characterized through various spectroscopic techniques, showcasing its potential in reductive carbonyl chemistry applications.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
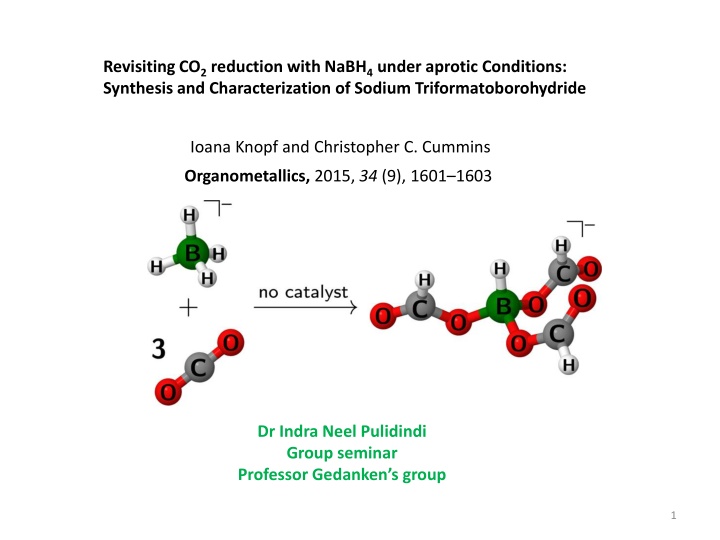
Revisiting CO2 reduction with NaBH4 under aprotic Conditions: Synthesis and Characterization of Sodium Triformatoborohydride Ioana Knopf and Christopher C. Cummins Organometallics, 2015, 34 (9), 1601 1603 Dr Indra Neel Pulidindi Group seminar Professor Gedanken s group 1
Choice of reductant, not the catalyst, is key to CO2 reduction CO2 hydroboration methods for formate production are becoming popular Eventhough hydroboration processes are catalytic they require stoichiometric reductants like pinacolborane or catecholborane Boranes are more expensive and less reactive NaBH4 is a famous reducing agent with large annual production and rich reductive Carbonyl chemistry The paucity of chemical literature on the reactivity of NaBH4 with CO2 is surprising NaBH4 reacts with 3 equivalents of CO2 in the absence of a catalyst 2
Reaction of NaBH4 with CO2 under aprotic conditions Sparging a solution of NaBH4 is acetonitrile (Schlenk flask) with a stream of CO2 at room temp. for 10 min. turned the solution cloudy and resulted in the formation of a white precipitation The precipitate was found to be a mixture of Na[HB(OCHO)3] and Na[H2B(OCHO)2] no change in ratio between Na[H2B(OCHO)2] and Na[HB(OCHO)3] was observed after keeping the solid mixture under vacuum for prolonged periods of time 3
Synthesis of pure Na[HB(OCHO)3] Solution of NaBH4 in acetonitrile in a Parr vessel was pressurized with CO2 (20 atm). The reaction was carried out under stirring for 1.5 h. The precipitate obtained was dried under vacuum. a signal at = 8.28 ppm is characteristic of formyl protons 13C satellites corresponding to 1JCH = 210 Hz A signal at = 3.79 ppm is characteristic of borohydride proton with four-line splitting pattern with 1JBH = 131 Hz 1H NMR spectrum of Na[HB(OCHO)3] in CD3CN at 25 C 4
Exclusive formation of triformatoborohydride, Na[HB(OCHO)3] 11B NMR spectrum of Na[HB(OCHO)3] in CD3CN at 25 C 13C NMR spectrum of Na[HB(OCHO)3] in CD3CN at 25 C 11B NMR spectrum is diagnostic a single at 165.5 ppm is typical of formate carbon resonance A clear doublet with a B H coupling constant of 130 Hz is observed 5
ATR-IR spectrum of Na[HB(OCHO)3] 2916 (C H), 2502 (B H), 1685 (C=O) cm-1 6
Structure of triformatoborohydride the first of its kind Single crystals of [Na(DME)][HB(OCHO)3] were obtained by cooling a saturated DME solution of Na[HB(OCHO)3] to 40 C The compound crystallized in the monoclinic space group P21/c The boron center is pseudotetrahedral, with an average B O interatomic distance of 1.48 Each sodium ion resides in a pseudo-octahedral environment with one DME molecule occupying two adjacent coordination sites, while the other four are filled by carbonyl oxygens from proximate formate groups Within each formate unit, the C O interatomic distances are inequivalent; for example, C1 O1 is 1.3060(14) and C1 O4 is 1.2073(14) , with an O4 C1 O1 angle of 126.50(10) No structurally characterized tricarboxylate borohydride Salts is found in the CSD making, thus making this structure of triformatoborohydride the first of its kind Solid-state structure of [Na(DME)][HB(OCHO)3] 7
Does Na+ played any role in CO2 reduction? Instead of NaBH4, [NEt4][BH4] is used as borohydride source Identical reaction conditions: 20 atm. of CO2 and dry acetonitrile as the solvent, stirring, 1 h Corresponding, [NEt4][HB(OCHO)3] , was produced in good purity Thus the CO2 reactivity is inherent of the borohydride anion The reaction does not require the presence of a strongly coordinating alkali metal cation (Na+) 8
Formation of [NEt4][HB(OCHO)3] : 8.29 (1 H, s), 3.79 (1 H, m, 1JBH= 130 Hz) ppm 1H NMR spectrum of [NEt4][HB(OCHO)3] in CD3CN at 25 C : 165.2 (s) ppm, resonance signal due to formate carbon 9 13C NMR spectrum of [NEt4][HB(OCHO)3] in CD3CN at 25 C
: 3.70 (d, 1JBH = 130 Hz) ppm 11B NMR spectrum of [NEt4][HB(OCHO)3] in CD3CN at 25 C Major signal with m/z value of 147, confirm the presense of [HB(OCHO)3]- anion in the compound ESI-MS spectrum (negative mode) of [NEt4][HB(OCHO)3] 10
Reaction of NaBH4 with CO2 in wet aerobic conditions Solutions of NaBH4 in wet acetonitrile were prepared in air with no special precautions CO2 was bubbled through the solution which resulted in immediate formation of white precipitate After 10 min. the reaction was quenched with 1 M aqueous HCl Formic acid (1.5 equiv) was produced, as quantified by 1H NMR spectroscopy In the absence of strictly anaerobic and anhydrous conditions, the full potential of NaBH4 to reduce 3 equivalents of CO2 could not be exploited owing to the completing NaBH4 hydrolysis reactions The average of 4 experiments was 1.51 equivalents of formic acid produced per equivalent of NaBH4 Typical 1H NMR spectrum of the reaction mixture with an internal standard in D2O at 25 C 11
Summary: Reduction of CO2 to formate is achieved easily using sodium borohydride without employing expensive boranes or intricate catalysts. This finding complements the recent report from Mizuta and co-workers describing the reduction of CO2 to methoxide with commercial BH3 THF. The reactivity of CO2 with borohydride is unperturbed by the use of a non-coordinating cation in lieu of sodium. Consequently, the intrinsic reactivity of the borohydride anion should always be taken into account and rigorously controlled for when studying borohydride-containing systems for substrate reduction 12